Article Text
Abstract
Background: Asthma exacerbation is the most common cause of hospital admission in children. A study was undertaken to investigate the importance of allergen exposure in sensitised individuals in combination with viral infections and other potentially modifiable risk factors precipitating asthma hospital admission in children.
Methods: Eighty four children aged 3–17 years admitted to hospital over a 1 year period with an acute asthma exacerbation (AA) were matched for age and sex with two control groups: stable asthmatics (SA) and children admitted to hospital with non-respiratory conditions (IC). Risk factors were assessed by questionnaires and determination of allergen sensitisation, home allergen exposure, pollen exposure, and respiratory virus infection.
Results: Several non-modifiable factors (atopy, duration of asthma) were associated with increased risk. Among the modifiable factors, pet ownership, housing characteristics, and parental smoking did not differ between the groups. Regular inhaled corticosteroid treatment was significantly less common in the AA group than in the SA group (OR 0.2, 95% CI 0.1 to 0.6; p = 0.002). A significantly higher proportion of the AA group were virus infected (44%) and sensitised and highly exposed to sensitising allergen (76%) compared with the SA (18% and 48%) and IC groups (17% and 28%; both p<0.001). In a multiple conditional logistic regression (AA v SA), allergen sensitisation and exposure or virus detection alone were no longer independently associated with hospital admission. However, the combination of virus detection and sensitisation with high allergen exposure substantially increased the risk of admission to hospital (OR 19.4, 95% CI 3.7 to 101.5, p<0.001).
Conclusions: Natural virus infection and real life allergen exposure in allergic asthmatic children increase the risk of hospital admission. Strategies for preventing exacerbations will need to address these factors.
- AA, acute asthma
- IC, inpatient control
- OR, odds ratio
- PCR, polymerase chain reaction
- PG, pollen grain
- SA, stable asthma
- asthma
- inhaled allergens
- viruses
- atopy
- children
- hospitalisation
Statistics from Altmetric.com
- AA, acute asthma
- IC, inpatient control
- OR, odds ratio
- PCR, polymerase chain reaction
- PG, pollen grain
- SA, stable asthma
Asthma is one of the common causes of acute admission to hospital in children. Current treatments, although helpful, are still unable to prevent childhood asthma exacerbations completely.1 In order to improve asthma management it is essential to understand clearly the causes of exacerbations leading to hospital admission which are amenable for intervention. Previous admission to hospital with asthma2,3 and sensitisation to indoor allergens increase the risk of hospital admission,4,5 but neither is modifiable. The association between virus infections and exacerbation of asthma in children was initially demonstrated using culture and serological methods,6 and subsequently confirmed by similar or more modern techniques.7,8,9,10,11 Viruses were detected using polymerase chain reaction in ∼80% of asthma exacerbations in children.12 One of the other factors associated with increased emergency room visits and hospital admissions with asthma attacks in children is high exposure to allergens.13,14 Experimental data suggest a synergistic interaction between allergens and viruses.15
Although the interactions of allergen sensitisation with virus infection4 and with high exposure to specific allergen5 have been reported previously, no study to date has investigated the relationship between all three factors in childhood exacerbations. We carried out a case-control study investigating the importance of exposure in sensitised individuals in combination with viral infection precipitating acute asthma attacks resulting in hospital admission in children. We also investigated other risk factors that are potentially modifiable in order to help determine where preventive strategies should be focused in the future.
METHODS
Participants
The study was carried out in the South Manchester University Hospital over a 12 month period. Children aged 3–17 years admitted to hospital with an acute asthma exacerbation (asthma admission, AA) were matched for age (±2 years) and sex with two control groups: (1) patients with stable asthma who were not admitted to hospital and did not require oral steroids for asthma exacerbation within the previous 12 months (stable asthma, SA; recruited from the outpatient department); and (2) patients admitted to hospital with non-respiratory conditions (inpatient control, IC). Controls were enrolled within 3 weeks of recruitment of the index case.
The study was approved by the local research ethics committee and informed consent was obtained from all parents (and children when appropriate).
Outcomes
Information was collected on medication use, housing conditions, family history, parental smoking, pet ownership, and previous hospital admissions using validated respiratory and environmental questionnaires.16
Sensitisation status was ascertained using skin prick testing (Dermatophagoides pteronyssinus, cat, dog, mixed grasses, negative and positive controls; Bayer, Elkahrt, IN, USA). Sensitisation was defined as a weal diameter 3 mm greater than negative control. We also measured total and specific serum IgE (mite, cat, dog, ryegrass; UniCAP, Pharmacia, Uppsala, Sweden) and defined sensitisation as a concentration of allergen specific IgE of >0.35 kU/l.
Nasal lavage fluid for virus detection was collected within 24 hours of recruitment using a 12 Fr balloon catheter. The tube was inserted into the nostril, the balloon inflated, and 2 ml sterile saline instilled for 30 seconds and then aspirated. The lavage fluid was mixed with 2 ml sterile viral culture medium, immediately frozen on dry ice, and stored at −70°C. Samples were analysed by polymerase chain reaction (PCR) for picornaviruses (rhinoviruses and enteroviruses), coronaviruses, respiratory syncytial virus, influenza A and B, parainfluenza viruses 1–3, adenoviruses, Chlamydia and Mycoplasma pneumoniae. The methods were adapted from those published.17 Details of target genes, primer sequences, and cycling variables are available on request. All samples were analysed blinded to groups.
Indoor allergen levels (Der p 1, Fel d 1, Can f 1) were measured using monoclonal antibody based enzyme linked immunoassays in the dust samples taken from the child’s mattress and living-room floor, which were collected within 2 weeks of recruitment.
Grass pollen exposure was estimated using pollen counts (number of pollen grains per cubic metre of air sampled (PG)/m3) averaged over 24 hours) which were obtained from the UK Pollen Monitoring Network local site.
Statistical analysis
With 84 matched subjects, an odds ratio (OR) of 2.5 or more for the potential risk factor(s) could be detected with 85% power at a significance level of 5%, assuming an underlying exposure rate to any of the risk factors of 50%.
Comparisons of groups AA v SA and AA v IC with respect to individual risk factors were carried out using conditional logistic regression using Stata Version 6.0 (Stata Corp, College Station, TX, USA). This analysis gave appropriate adjustment for the one-to-one matching of the groups. In order to investigate the effect of individual or combinations of sensitisation, exposure to sensitising allergen and viral infection, a constructed variable was created which was stratified for the presence and combination of factors (sensitised only; virus detected only; sensitised and exposed only; sensitised and virus detected only; sensitised and exposed and virus detected (that is, mutually exclusive categories); reference category neither sensitised nor virus detected (± exposed)). Multiple conditional logistic regression analysis was then used to assess the significance of various factors adjusting for the influence of other variables which were shown to be significantly associated with the AA group in the univariate analysis. The results are presented as ORs with 95% confidence intervals (95% CI). ORs were calculated for both categorical (usually presence/absence) and continuous variables. For the former, the OR simply shows the increased or decreased risk of asthma admission with the presence of the risk factor; for the latter, the OR shows the multiplicative increased or decreased risk with each unit (or log unit) increase.
The problem of multiple comparisons, given the number of risk factors considered and the inherent increased likelihood of obtaining significant results by chance, is acknowledged. Hence, for the univariate analyses group differences are interpreted as being significantly different only for p values <0.01.
High exposure to dust mite, cat or dog allergen was considered when Der p 1 >2 μg/g,18Fel d 1 >8 μg/g,19 and Can f 1 >10 μg/g.20 These values were used to divide study participants into those “exposed” or “not exposed” to each allergen. The pollen count was given as low, moderate, high, or very high based on the following cut off levels: low <30 PG/m3; moderate 30–49 PG/m3; high 50–149 PG/m3; very high >150 PG/m3. Children were classified as exposed to grass pollen if the average pollen count for the 7 days before the date they were recruited was classed as high or very high.
RESULTS
Participants
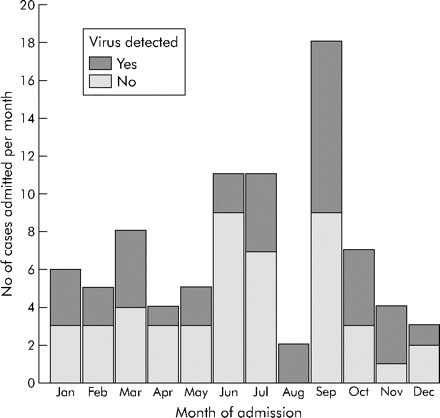
Eighty four of 125 children (55 (65.5%) boys, mean age 7.04 years) admitted to hospital for an asthma exacerbation between 1 February 2000 and 31 January 2001 were recruited into the study with matching controls. Of the 41 who did not take part, 10 parents declined to give informed consent and the remaining 31 were unable to give consent because the parents were not present in the hospital. There was no difference in age and sex between the children who took part and those who did not. Six participants (two boys, age range 3–16 years) were readmitted during the course of the study but were not included again. The largest number of admissions occurred in September (n = 18), followed by June and July (n = 11), and the fewest in August (n = 2).
Forty eight children in the SA control group had had at least one previous hospital admission with an asthma exacerbation compared with 59 in the AA group (table 1, p = 0.12). There was no significant difference in the number of previous asthma admissions between the two groups (p = 0.88).
Non-modifiable risk factors for hospital admission with acute asthma
Non-modifiable risk factors
The risk factors not amenable to intervention in the three study groups are shown in table 1. There were no significant differences between the groups in ethnicity. Children in both asthma groups (AA and SA) were more likely to have eczema and hay fever than children without asthma (IC), with no difference between AA and SA. The proportion of sensitised children differed significantly between the three groups with children in the AA group being more likely to be skin test positive to at least one allergen than those in the SA (OR 3.6, 95% CI 1.7 to 7.9, p = 0.001) or IC group (OR 16.3, 95% CI 5.1 to 52, p<0.001). This pattern was similar for each allergen but reached statistical significance between the AA and SA groups only for dog. Levels of total and allergen specific IgE were higher in the AA group, but this difference reached significance only between the AA and IC groups.
Modifiable risk factors
The potentially modifiable risk factors in the three study groups are shown in table 2. There were no significant differences between the groups in maternal or paternal smoking, pet ownership, housing characteristics, and deprivation index score. Regular inhaled corticosteroid (ICS) treatment was significantly less common in the AA group than in the SA group (OR 0.2, 95% CI 0.1 to 0.6, p = 0.002).
Potentially modifiable risk factors for hospital admission
Virus detection
Sixty nine children had a virus detected in their nasal washing (44% in the AA group, 17.9% in the SA group, and 16.7% in the IC group, p<0.001). Rhinoviruses were the most commonly detected respiratory pathogens accounting for 81% of viruses detected in the AA group; they were the only virus type detected more frequently in the AA group than the other groups (fig 1). Children in the AA group were significantly more likely to have a virus detected than those in the SA (OR 5.4, 95% CI 2.1 to 14.0, p = 0.001) or IC groups (OR 4.3, 95% CI 1.9 to 9.8, p = 0.001). Viruses were most commonly detected from August through to November, with levels of detection of 50–100% in children in the AA group in these months (fig 2).
Respiratory pathogens detected in the admitted asthmatic (AA), stable asthmatic (SA), and non-asthmatic inpatient control (IC) groups.
Number of asthma admissions per month and the proportion in whom a virus was detected.
Allergen exposure
Dust samples were obtained from both sampling sites in 250 homes. Samples were not available for two cases (home access not gained); these two cases and their controls were excluded from the exposure analyses. There were no differences in the concentrations of mite, cat and dog allergen in either of the sampling sites between the study groups (full data available on request). Grass pollen was only detectable from early May to mid October. However, levels of more than 50 PG/m3 (high or very high) were detected from early June to mid July (full data available on request). Children in the AA group were no more likely to be exposed to any one of the allergens than those in either the SA group (OR 1.6, 95% CI 0.5 to 4.9, p = 0.41) or IC group (OR 0.7, 95% CI 0.2 to 2.4, p = 0.53).
Combination of sensitisation and exposure to sensitising allergen
Children were classed by the presence of a positive skin test to an allergen (sensitised) and whether they were exposed to high levels of sensitising allergen (table 2). Children in the AA group were significantly more likely to be sensitised and exposed to at least one allergen than those in the SA (OR 2.9, 95% CI 1.5 to 5.6, p = 0.001) or IC groups (OR 10.8, 95% CI 3.9 to 29.9, p<0.001).
Combination of sensitisation and exposure to sensitising allergen and virus detection
A significant difference was observed between the groups with respect to the combination of sensitisation and exposure to at least one sensitising allergen and virus detection (35.4% in the AA group, 7.3% in the SA group, and 2.4% in the IC group, p<0.001). Children in the AA group were at a significantly higher risk of being sensitised and exposed to the sensitising allergen and having a respiratory pathogen detectable than those in the SA (OR 8.7, 95% CI 2.6 to 28.6, p<0.001) or IC group (OR 28.0, 95% CI 3.8 to 206, p = 0.001). A similar pattern was seen in AA children who were sensitised and exposed to the sensitising allergen and had rhinovirus present compared with SA children (OR 23.0, 95% CI 3.1 to 170, p = 0.002), although confidence intervals were large due to the smaller numbers of children (table 2).
In order to establish the ORs of individual and combined factors, children were assigned to specific groups within a constructed variable adjusting for the presence or absence of individual factors (sensitised only, virus only, sensitised and exposed only, sensitised and virus only, sensitised and exposed and virus). Children in the AA group were at a significantly higher risk of being sensitised and exposed to the sensitising allergen and having a virus detectable than children in the SA group (OR 22.7, 95% CI 4.6 to 112.5, p<0.001). However, other individual or combination variables failed to reach significance (table 3).
Odds ratios (95% CI) for risks factors for hospital admission using constructed variable* (mutually exclusive catergories), univariate and multivariate (adjusted for use of ICS and duration of asthma)
Multivariate analysis of risk factors for hospital admission
Further analysis of risk factors for hospital admission within the two groups of asthmatic patients (AA and SA) was carried out using multiple conditional logistic regression adjusting for other risk factors that had been significant in the univariate analysis (use of inhaled corticosteroids and duration of asthma symptoms). The combination of sensitisation and exposure to high levels of sensitising allergen and virus detection remained a significant independent risk factor for hospital admission (OR 19.4, 95% CI 3.7 to 101.5, p<0.0001; table 3). The regular use of inhaled corticosteroids had a protective effect on hospital admission (OR 0.3, 95% CI 0.1 to 0.9, p = 0.03) whereas duration of asthma no longer affected admission (OR 0.9, 95% CI 0.8 to 1.1, p = 0.3).
DISCUSSION
Admission to hospital is an important risk factor for death from asthma.21 In addition, much of the cost that asthma inflicts on society (1–2% of the total health budgets in direct costs) results from hospital admissions. In spite of this, little is known about the causes of acute asthma exacerbations resulting in hospital admission. Knowledge of the potentially modifiable risk factors is essential for the development of strategies to prevent admissions. In this study we report that hospital admissions in children with asthma exacerbations are associated with a combination of both sensitisation and current high exposure to sensitising allergen and the presence of virus infection. Their combined effect is greater than the individual effects, suggesting a possible synergism between virus infection and allergen exposure in sensitised patients.
In this study we also identified regular use of an inhaled corticosteroid as a protective factor. Benefits from regular inhaled corticosteroids in preventing exacerbations in children with persistent asthma have been clearly demonstrated.22,23 Similarly, regular use of low dose inhaled corticosteroids is associated with a decreased risk of death from asthma in 5–44 year old patients.24 However, it has to be emphasised that there are no reported benefits from regular inhaled corticosteroids in patients only with intermittent virus induced wheezing,1,25 and it is likely that at least a component of virus induced wheezing is relatively resistant to steroids.1
Comparison with other clinical studies
In agreement with some previous studies,4,19 several non-modifiable risk factors including the degree of allergen sensitisation were associated with an increased risk of hospital admission with asthma. Several studies have examined the role of respiratory viruses in the exacerbation of asthma leading to hospital admissions or attendance at the emergency room.4,26–29 In agreement with our study, a higher rate of virus detection has been reported in children who were admitted to hospital compared with either asthmatic or non-asthmatic controls. Although most of these studies reported a higher frequency of virus detection than in the current study, many have included younger children of less than 3 years.4,26,27 Studies which recruited older children of a comparable age reported a similar virus detection rate to ours.28,29
Allergen exposure has been related to asthma severity in sensitised individuals.5,14 Sporik et al reported that, among children admitted to hospital with an acute asthma attack, those who were sensitised and exposed to dust mite had an increased risk of readmission during the following month.14 In our study, six children were readmitted during the 12 month study period. Each of these children was sensitised and exposed to at least one allergen, and three of them were sensitised and exposed to more than one allergen. In the US Inner City Asthma Study, children who were sensitised and highly exposed to cockroach allergen were more likely to be admitted to hospital, had more unscheduled medical visits, and more time off school than either non-exposed or non-sensitised children.5 However, in the Childhood Asthma Management Program Study, children sensitised to dog and exposed to high levels of dog allergen were more likely to be admitted to hospital in the univariate analysis but not in the multivariate analysis.30
The results of our univariate analysis show that both virus infection and high exposure to inhalant allergens in sensitised individuals are significant risk factors for hospital admission. However, in the multivariate analysis their individual effects were no longer significant, but the combination of these risk factors increased the risk of hospital admission almost 20-fold. In a recent study of similar design in adults we found that the risk of hospital admission was also markedly increased (about sixfold) with the combination of sensitisation and current exposure to high levels of sensitising allergens and the presence of virus infection.31 It is worth noting that the effect of viruses on sensitised individuals who are exposed to allergen appears much greater in children.
Experimental studies
Several experimental studies have suggested an interaction between allergens and viruses when individuals are exposed to both factors simultaneously.15,32,33 Patients with ragweed allergic rhinitis have a significant increase in airway reactivity to both histamine and ragweed antigen when infected with rhinovirus, and a significant increase in late phase response after virus inoculation.32 Bronchoalveolar lavage fluid obtained from patients with allergic rhinitis both during the acute virus infection and 1 month later showed significantly enhanced histamine release, tumour necrosis factor α, and greater recruitment of eosinophils to the airway 48 hours after local antigen challenge.15,33 These changes were not seen in either non-allergic volunteers infected with rhinovirus and challenged with antigen, allergic individuals challenged with antigen prior to infection, or in allergic individuals infected with virus and sham challenged with saline. Thus, a proposed mechanism for the synergistic effect observed when a sensitised and allergen exposed asthmatic becomes infected with a virus is a synergistic augmentation in pro-inflammatory pathways in the airway which results in augmented bronchial inflammation. The precise mechanisms of this process require further detailed investigation.
In contrast to the above studies in which an interaction was observed, a recent study investigating the effect of inhaling low dose mite allergen for 10 days prior to experimental rhinovirus infection in adults with mild asthma failed to observe any synergism.34 However, patients in this study were not exposed to allergen and infected with virus concurrently, and this may explain why there was no interaction in any of the clinical or inflammatory outcomes. These differing experimental data emphasise the importance of carrying out studies on patients following natural infection and real life exposure to allergens.
Limitations of the study
Ideally, a prospective population based study would be the best study design to evaluate risk factors for hospital admission with an asthma exacerbation, but clearly this would be very difficult and time consuming and therefore a case-control study design was used for practical reasons.
Not all children who were admitted with an exacerbation during the 12 month period of the study took part. Consent was obtained from approximately 67% of parents and children. However, only 10 parents declined to give informed consent, and the remaining 31 were unable to give consent because the parents were not present in the hospital. This may have introduced some selection bias into the study. We have no information regarding the nature or severity of asthma in these children. It is important to note that there was no difference in age and sex between children who took part and those who did not.
Within the study no attempt was made to match the asthma cases and controls for severity of asthma. However, all the stable asthma controls were recruited from hospital outpatients so their asthma was sufficiently severe to require monitoring in a secondary care setting and not by their primary care physician alone. We were unable to use lung function tests to match AA and SA children as many of them were too young to perform spirometric tests (median age 7 years), and spirometry was not routinely carried out on all patients in the hospital outpatient department. Thus, it may be that some of the findings are not due to the risk factors attributed to them but result from differences in severity of asthma between cases and controls. However, we believe that our cases and controls are relatively well matched for severity as there are similar numbers of children in each group with daily wheeze symptoms, who have been admitted to hospital before with an asthma exacerbation, and the median number of previous admissions was similar in the two groups.
It is also of importance to note that we were unable to carry out a formal statistical test for an interaction between virus infection, allergen exposure, and sensitisation due to the size of the study. We estimate that we would need several thousand children in the study to perform such a three way interaction test. Thus, although our results suggest an additional effect of the combined factors compared with the individual factors, we cannot be sure of a synergistic effect given the tests we have been able to carry out.
Conclusions
Among potentially modifiable factors, admission to hospital with acute asthma was strongly associated with the combination of sensitisation and current exposure to high levels of sensitising allergens and the presence of virus infection. Neither of these factors alone—nor parental smoking, pet ownership and housing characteristics—increased the risk for hospital admission. Regular inhaled corticosteroid use was significantly associated with a reduced risk for hospital admission. These results indicate that there appears to be a combined rather than an individual effect of natural virus infection and real life allergen exposure in allergic asthmatic children in inducing asthma exacerbations resulting in hospital admission. Strategies to reduce the impact of hospital admission of children with asthma exacerbations should be focused on both the reduction of allergen exposure in sensitised individuals and the development of effective therapies for the prevention or treatment of virus infections.
Acknowledgments
The authors thank all the parents and children who took part in the study and all the paediatric staff at South Manchester University Hospital for allowing them to recruit their patients. Pollen exposure data were kindly supplied by Pollen UK local network monitoring site.
REFERENCES
Footnotes
-
Published Online First 29 December 2005
-
Financial support of viral PCR work was by The British Lung Foundation/Severin Waterman Family Foundation Lung Research Programme (grant number P00/2).
-
Competing interests: none declared.