Article Text
Abstract
Background: The low oxygen environment during air travel may result in hypoxia in patients with respiratory disease. However, little information exists on the oxygen requirements of infants with respiratory disease planning to fly. A study was undertaken to identify the clinical factors predictive of an in-flight oxygen requirement from a retrospective review of hypoxia challenge tests (inhalation of 14–15% oxygen for 20 minutes) in infants referred for fitness to fly assessment.
Methods: Data from 47 infants (median corrected age 1.4 months) with a history of neonatal lung disease but not receiving supplemental oxygen at the time of hypoxia testing are reported. The neonatal and current clinical information of the infants were analysed in terms of their ability to predict the hypoxia test results.
Results: Thirty eight infants (81%) desaturated below 85% and warranted prescription of supplemental in-flight oxygen. Baseline oxygen saturation was >95% in all infants. Age at the time of the hypoxia test, either postmenstrual or corrected, significantly predicted the outcome of the hypoxia test (odds ratio 0.82; 95% confidence intervals 0.62 to 0.95; p = 0.005). Children passing the hypoxia test were significantly older than those requiring in-flight oxygen (median corrected age (10–90th centiles) 12.7 (3.0–43.4) v 0 (−0.9–10.9) months; p<0.0001).
Conclusions: A high proportion of ex-preterm infants not currently requiring supplemental oxygen referred for fitness-to-fly assessment and less than 12 months corrected age are at a high risk of requiring in-flight oxygen. Referral of this patient group for fitness to fly assessment including a hypoxia test may be indicated.
- Fio2, fractional inspired oxygen
- nCLD, neonatal chronic lung disease
- PMA, postmenstrual age
- Spo2, pulse oxygen saturation
- infants
- fitness to fly
- hypoxia
- air travel
- neonatal chronic lung disease
Statistics from Altmetric.com
- Fio2, fractional inspired oxygen
- nCLD, neonatal chronic lung disease
- PMA, postmenstrual age
- Spo2, pulse oxygen saturation
The number of people including children using commercial aircraft to travel is increasing. Commercial airlines generally cruise between 9150 and 13 000 metres above sea level.1 For passenger safety during flight at these altitudes, commercial aircraft are pressurised to maintain a cabin pressure equivalent to 1530–2440 metres. As the altitude increases the partial pressure of oxygen in the atmosphere falls, so passengers in aircraft at cruising altitude are breathing the equivalent of 15–16% of fractional inspired oxygen (Fio2) at sea level.1 This lower oxygen environment elicits little or no clinically relevant effects in healthy adults but may result in a lowered arterial haemoglobin oxygen saturation.2,3 Lee and coworkers4 studied healthy children during air travel and reported mean pulse oxygen saturations (Spo2) decreasing from 98% at sea level to 94–95% during commercial air travel without notable clinical symptoms. However, patients with pre-existing respiratory conditions such as chronic obstructive pulmonary disease, cystic fibrosis, neonatal chronic lung disease (nCLD), or other chronic lung diseases may develop hypoxia related respiratory distress leading to symptom exacerbation, altitude related illnesses, or even death during flights.1,5,6
In 2002 the British Thoracic Society (BTS) issued recommendations for passengers with respiratory disease planning air travel.7 These guidelines provide useful information for the screening of patients with respiratory disease. However, while the guidelines for adults are well supported by studies in the medical literature, the same is not true for children. The updated BTS air travel guidelines (www.brit-thoracic.org.uk) suggest that a pre-flight hypoxia test should be performed in older children with chronic lung disease and forced expiratory volume in 1 second (FEV1) of less than 50% predicted. Recommendations for young children who are unable to perform spirometric tests are that infants should wait 1 week after birth before being allowed to fly, while infants with a history of neonatal respiratory disease should consult a paediatrician and a hypoxia test should be considered.7 The 2002 BTS guidelines suggest that adults with respiratory disease and a room air Spo2 of 92–95% should undergo a hypoxic challenge, while those with Spo2 <92% should receive in-flight oxygen. Patients undergoing a hypoxia test are considered to require in-flight oxygen if the Spo2 falls below 85% during the test.7 The 2004 BTS update of these guidelines suggested that a Spo2 of <90% was indicative of an in-flight oxygen requirement in young children and infants with a history of respiratory disease; recommendations for adults remained unchanged.
The hypoxia test is a simple method of demonstrating a passenger’s need for supplemental oxygen during flight and for determining in-flight oxygen requirements. Data on the use of hypoxia tests in infants and older children are sparse. The hypoxia test predicted cases of oxygen desaturation during flight in children with cystic fibrosis,8 but pre-flight spirometry predicted oxygen desaturation during flight better than hypoxia testing in those old enough to perform formal lung function tests.9 We are aware of only two studies that used hypoxia tests in infants and young children. Parkins et al10 performed sleep studies in 34 healthy infants aged 1–6 months during a prolonged hypoxia test and reported that the mean Spo2 declined from 97.6% to 92.8%. Interestingly, four infants had significant oxygen desaturation below 80%. More recently, Buchdahl et al11 reported the use of hypoxia tests in a case series of 20 young children with a history of respiratory disease; six patients with Spo2 ⩾95% in room air desaturated below 90% when performing the hypoxia test.
Thus, most healthy infants and young children do not appear to exhibit clinically relevant oxygen desaturation during air travel4 or following a hypoxia test.10 However, some healthy infants10 and infants and young children with respiratory disease may be at risk of significant hypoxic events.11
The Women’s and Children’s Health Service, comprising King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, provides the only tertiary obstetric, neonatal and paediatric medical service in the state of Western Australia. This study reports the results of a retrospective review of infants and young children with a history of neonatal lung disease referred for a pre-flight hypoxia test. We aimed to identify those neonatal factors that may be predictive of a requirement for in-flight oxygen in infants with a history of nCLD.
METHODS
Subjects
All children referred to the Respiratory Function Laboratory of Princess Margaret Hospital for Children, Perth, Western Australia for a hypoxia challenge test from January 2000 to December 2003 were identified. During this period approximately 280 children per annum were born with a gestational age of less than 36 weeks. Patients were referred by neonatal or paediatric specialists for assessment of fitness to fly prior to planned air travel, usually for transfer from tertiary neonatal and paediatric facilities to rural and regional medical units.
Forty seven infants and young children were identified and the medical records and hypoxia test results of these children were retrospectively reviewed. Children were free of respiratory infections at the time of testing and all had a history of neonatal respiratory distress and were further categorised as either nCLD or non-nCLD. Neonatal CLD was defined as the use of supplemental oxygen at 36 weeks postmenstrual age (PMA) for infants with a gestational age at birth of <32 weeks and the use of supplemental oxygen at 28 days of life for individuals with a gestational age at birth of ⩾32 weeks.12
The neonatal and clinical data collected were: sex, gestational age and weight at birth, delivery mode, Apgar scores at 5 minutes, duration of mechanical ventilation and continuous positive airway pressure (CPAP), and days of supplemental oxygen in hospital and at home. The days since discontinuation of supplemental oxygen, PMA, corrected age (calculated as PMA – 40), and body weight at the time of the hypoxia test were also recorded.
Ethical approval to conduct the review and publish the results was obtained from the Princess Margaret Hospital Medical ethics committee.
Procedure for hypoxic challenge test
The following procedure was used for assessing the requirement for in-flight oxygen. Before commencing the hypoxia test the children were fitted with a nasal cannula to allow oxygen administration if required. Baseline Spo2 and pulse rate (NPB-395, Nellcor) while breathing room air over a 2 minute period were recorded.
Children were then exposed to high flow (15 l/min) 14% oxygen in nitrogen (Air Liquide Healthcare, Australia) via a non-rebreathing mask incorporating a one way valve assembly (Model 1058, Hudson) for a period of 20 minutes. The face mask does not provide a leak free seal, but the high flow 14% oxygen acts to surround the patient’s face in a low oxygen environment. Previous work in our laboratory has shown that this method maintains a Fio2 of 14–15% measured at the nares for the duration of the study (unpublished data).
Clinical observations including Spo2, pulse rate, and activity state (crying, settled, asleep) were recorded at 1 and 20 minutes and between the following times: 3–4 minutes, 5–7 minutes, 10–12 minutes, and 15–17 minutes. A patient was considered as fit to fly without supplemental oxygen if Spo2 remained above 85% for the duration of the test. If Spo2 fell below 85%, the child was considered to have failed the hypoxia test and to require in-flight oxygen. At this point oxygen was administered through the nasal cannula commencing at 0.125 l/min with increments every minute to 0.25, 0.5 and 1 l/min until Spo2 exceeded 94–96%. Once Spo2 exceeded 94–96%, the infant was monitored for a further 5 minutes to ensure oxygen saturation levels remained stable. None of the infants studied required supplemental oxygen at a rate of more than 1 l/min to achieve adequate oxygenation.
Statistical analysis
Independent variables for predicting the results of the hypoxia test in the total study population were assessed as follows. Each independent variable was analysed in terms of its ability to predict the outcome of the hypoxia test (pass or fail) using binary logistic regression. Predicting factors significantly associated with the hypoxia test result in the binary logistic regression analysis were then included in multiple logistic regression analyses. Data are expressed as median and 10–90th centiles. The Mann-Whitney U test was used to compare significant univariate predicting factors between nCLD infants who passed or failed the hypoxia test. A p value of <0.05 was considered statistically significant. All analyses were performed using SPSS software Version 11.5.
Sensitivity and specificity of different corrected ages for predicting hypoxia test results were calculated and used to construct a receiver operating characteristic curve to define the optimum cut off age by plotting sensitivity and 1 − specificity against possible cut off values.
RESULTS
Forty seven infants (32 with nCLD) were referred for testing during the period of the review and were included in the analyses. The median age (10–90th centiles) of the study population was 46.0 (36.8–113.6) weeks PMA, equivalent to a corrected age of 1.4 (−0.7–17.0) months. All infants studied had baseline Spo2 >95% (range 95–100%). The majority of infants tolerated the hypoxia test well. In the period immediately before discontinuing the test, 27 of 32 infants with nCLD were awake and quiet, the remainder being asleep (n = 2) or awake and crying or restless (n = 3). All 15 infants without nCLD were awake and quiet. Demographic and hypoxic test data are shown in table 1. Nine infants were classified as not requiring in-flight oxygen and were all in the nCLD group.
Demographic characteristics and hypoxic test results of the study population
Total study population
Statistically significant predicting factors for failing the hypoxia test were age and body weight at the time of testing, duration of receiving supplemental oxygen at home, and the time since discontinuing supplemental oxygen before the hypoxia test (p<0.05, table 2). These factors, other than body weight, were assessed using multiple logistic regression analysis. Body weight was excluded because of significant co-linearity with age (r = 0.97, p<0.01). In the multiple logistic regression model, age at the time of the hypoxia test (either PMA or corrected) remained a significant predictor of the hypoxia test result (p = 0.005; PMA (weeks): odds ratio (OR) 0.96, 95% confidence interval (CI) 0.93 to 0.99; corrected age (months): OR 0.82, 95% CI 0.67 to 0.95). In contrast, duration of home oxygen (OR 1.002, 95% CI 0.985 to 1.02; p = 0.82) and time since oxygen administration (OR 1.006, 95% CI 0.996 to 1.016; p = 0.25) did not contribute to the ability to predict the result of the hypoxia test.
Results of univariate logistic regression analysis of total study population
Infants failing the hypoxia test were significantly younger than those not requiring in-flight oxygen (40.0 (36.0–87.1) and 95.0 (53.0–228.0) weeks PMA, respectively; p<0.0001) with corrected ages of 0.0 (−0.9–10.9) and 12.7 (3.0–43.4) months, respectively (p<0.0001). The median time for Spo2 to fall below 85% in infants failing the hypoxia test was 2 (1–5) minutes and was related to the number of days since supplemental oxygen was discontinued (r = 0.504; p = 0.001). Those infants failing the hypoxia test required a median (10–90th centiles) supplemental oxygen flow of 0.25 (0.113–0.5) l/min to return to Spo2 levels exceeding 94–96% while receiving 14% oxygen.
Neonatal chronic lung disease group
Infants with nCLD who passed the hypoxia test were significantly older and had been off supplemental oxygen for longer than those without nCLD, while the duration of home oxygen use in the two groups was not different (table 3). Multiple logistic regression including age at the time of testing (either PMA or corrected) and days since oxygen use showed that age at the time of testing was the primary characteristic influencing the outcome of the hypoxia test (PMA (weeks): OR 0.954, 95% CI 0.913 to 0.997, p = 0.035; days off oxygen: OR 1.005, 95% CI 0.996 to 1.013, p = 0.29).
Comparison of infants with nCLD who passed or failed the hypoxia test
Receiver operator characteristic curve (ROC)
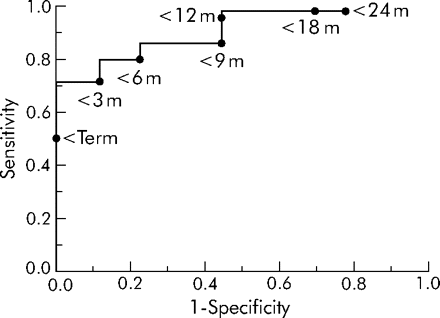
The sensitivity and specificity of specific corrected ages (term, 3, 6, 9, 12, 18 and 24 months) for predicting those infants failing the hypoxia teat were calculated and used to construct a receiver operator curve (ROC). The optimum corrected age for predicting an in-flight oxygen requirement was less than 12 months with a sensitivity of 95% and specificity of 56% (fig 1).
Receiver operator characteristic curve showing that the optimum corrected age for predicting the hypoxia test result was a corrected age of <12 months with a sensitivity of 95% and a specificity of 56%.
DISCUSSION
This retrospective review of infants referred for pre-flight hypoxia tests with a history of neonatal respiratory problems shows that a high proportion of infants (81%) exhibit significant oxygen desaturation below 85% when breathing a hypoxic gas mixture (Fio2 = 0.14). Interestingly, all infants had normal oxygen saturations (Spo2 >95%) in room air, suggesting that baseline Spo2 is not a useful screening tool for in-flight oxygen requirement in this patient group.
The BTS guidelines7 recommend that in-flight oxygen is not required in adults in whom sea level Spo2 is >95% or 92–95%, depending on the absence or presence of additional risk factors. Buchdahl et al11 reported their experience of pre-flight hypoxia testing in 20 young children with a mixture of chronic lung diseases. Eighteen infants and young children had normal baseline Spo2. Of these, six individuals exhibited oxygen desaturation below 90% with one infant recording a Spo2 of <85% during exposure to 15% oxygen. The present data support these earlier findings and suggest that a normal Spo2 in room air in infants and young children with a history of neonatal respiratory disorders is insufficient to determine the safety of this patient group in the low oxygen environment encountered during flight or at high altitude.
In the present study age at the time of testing (either PMA or corrected), irrespective of disease severity at the time of the hypoxia test, significantly predicted the requirement for in-flight oxygen. The median age of infants without nCLD referred for hypoxia testing was 37 weeks PMA, significantly younger than the median corrected age of infants who passed the test (12.7 months). In addition, all infants with a corrected age of less than 3 months failed the hypoxia test. This result may explain the failure of young infants without nCLD to pass the hypoxia test despite the fact that one might expect these infants to have less severe initial lung disease. The reasons why younger infants are more susceptible to hypoxia and thus fail the hypoxia test are not clear, but may be due to the relative immaturity of the respiratory system leading to increased ventilation-perfusion mismatch.1,13 In addition, the response to the hypoxic challenge in infants with a history of premature birth is liable to be more pronounced in the first months of life because of the influence of lung damage resulting from mechanical ventilation and oxygenation in the neonatal period. Furthermore, the preterm children without nCLD in the present study all had respiratory distress in the neonatal period. We are therefore cautious about extrapolating the conclusions from our observations to preterm children in general, although PMA appears to be a critical independent determinant of failure of the hypoxia test in our population.
Using ROC analysis, we found that infants of less than 12 months corrected age are significantly more likely to fail the hypoxia test when conducted in accordance with current recommendations (p<0.001). Thus, a pre-flight hypoxia test may be indicated in infants with a history of premature birth of less than 12 months corrected age. Additionally, based on current recommendations, all infants in the present study with a corrected age below 3 months would require in-flight oxygen, which suggests that young infants with a history of neonatal lung disease should not undertake air travel without supplemental oxygen before performing a pre-flight hypoxia test.
We routinely use a 20 minute hypoxia challenge for the assessment of fitness to fly in infants and young children. The hypoxia test, as used by our department, has a Spo2 lower limit of 85% for the administration of in-flight oxygen. While this differs from the current BTS recommendations of a 90% limit, it is in agreement with the original BTS guidelines7 and reported practices of other tertiary paediatric units.11 Altering the lower limit for prescription of in-flight oxygen from 85% to 90% is likely to increase the proportion of infants with a history of premature birth defined as requiring in-flight oxygen. While the relevance and accuracy of the hypoxia test has been tested in adults and adolescents,7 this is not the case in infants and young children. We use a face mask and non-rebreathing valve to deliver a high flow of 14% oxygen, and this differs from the approach suggested by the BTS guidelines.7 However, neither approach has been validated with subsequent measurements of in-flight Spo2, so we are unable to comment on the clinical validity of the hypoxia test as we use it to predict the actual in-flight oxygen requirement. Furthermore, the current study did not seek to evaluate clinical symptoms and oxygen saturation during flying. We therefore cannot draw conclusions as to the clinical relevance of the hypoxia test in infants with neonatal lung disease. Prospective studies comparing pre-flight hypoxia test results and clinical and oxygen status during flying in young children are required. In addition, disease specific studies are needed, as different diseases with differing pathophysiologies may result in distinctive responses to the hypoxia test.
In conclusion, a high proportion of ex-preterm infants not currently requiring supplemental oxygen referred to us for assessment of fitness-to-fly had significant oxygen desaturation when exposed to 14–15% oxygen. Critically, baseline oxygen saturation was normal in all infants, indicating that Spo2 in room air is not predictive of in-flight oxygen requirements in infants with a history of neonatal lung disease. Age at the time of the hypoxia test was a significant predictor of the test outcome, suggesting that infants and young children with a history of neonatal respiratory problems under 12 months corrected age may require in-flight oxygen during flight and should undergo a fitness to fly test before considering air travel. Further information is required to determine the effect of prematurity alone on hypoxia test results, and the clinical significance of failing current guidelines for safety during flight for infants with a history of neonatal lung disease.
REFERENCES
Footnotes
-
Published Online First 31 January 2006
-
KU was funded by the Siriraj Hospital, Thailand. PDS and SS are funded by the National Health and Medical Research Council, Australia.
-
Competing interests: none.