Article Text
Abstract
Objective To examine temporal trends in the incidence of epilepsy recorded in UK primary care and to evaluate the impact of recent efforts to improve the specificity of diagnosis in children.
Design Birth cohort study using primary care data from The Health Improvement Network, which includes a representative sample of the UK population of approximately 5%.
Methods We identified epilepsy recorded in primary care using relatively specific through to relatively sensitive indicators to identify epilepsy. Incidence estimates were based on 344 718 children aged 0–14 years with 1 447 760 years’ follow-up between 1994 and 2008. Trends in cumulative incidence were explored with stratified analysis by year-of-birth. Trends in annual incidence were investigated using Poisson regression with adjustment for age, gender and deprivation.
Results Cumulative incidence of recorded epilepsy at age 5 years ranged from 0.38% to 0.68% and annual incidence ranged from 71 to 116/100 000 person-years-at-risk, depending on the indicator used to identify epilepsy. With the most specific indicator for epilepsy, cumulative incidence was 33% lower among children born in 2003–2005 than in children born in 1994–1996, and annual incidence declined by 4% per annum between 2001 and 2008, after adjusting for age, gender and deprivation. Using a more sensitive indicator for epilepsy, the equivalent declines were 47% in cumulative incidence and 9% in annual incidence.
Conclusions The decline since the mid-1990s in epilepsy recorded in primary care may be due to more specific diagnosis, cessation of treatment for some forms of epilepsy, reduced exposure to risk factors or all of these factors.
- Epidemiology
- epilepsy
- United Kingdom
- incidence trends
- primary-care databases
Statistics from Altmetric.com
What is already known on this topic
-
UK estimates of the incidence of paediatric epilepsy may no longer be accurate due to the changes in environmental risk factors and changes in approaches to epilepsy diagnosis and treatment.
-
There have been reports of a decline in the incidence of paediatric epilepsy from two Nordic countries.
What this study adds
-
Up-to-date estimates of the annual and cumulative incidence of paediatric epilepsy recorded in UK primary care.
-
Evidence for a decline in paediatric epilepsy recorded in primary care in the UK.
-
Corroboration of the recent and ongoing declines in Europe of recorded paediatric epilepsy.
Introduction
Epilepsy is a serious neurological disorder in childhood and has long-term implications for health and well-being. It is a disorder with a relatively high prevalence and high seriousness (with attendant risks of early mortality and comorbidity). The UK incidence estimates are based on data collected in 1980–1990s, where the incidence ranged between 35 and 190/100 000 children.1–6 These existing estimates may no longer be relevant due to changes in early childhood risk factors (eg, meningitis and intracranial injuries). Changing approaches to diagnosis may also have had an impact with the release in 2004 of national guidelines focused on reducing misdiagnosis.7–9
Evidence of reducing rates of childhood epilepsy recorded in healthcare has been reported in two recent longitudinal studies in Nordic countries, where declines were found in antiepileptic drug (AED) prescriptions and hospitalisations for epilepsy.10 ,11 Information on trends in the UK is limited to one study showing a decline in prescription for AEDs in children between 1993 and 2005.12 The extent to which these findings were explained by chance, a shift in the age at diagnosis or reduced rates of treatment for children with recorded epilepsy, was not explored.
In this study, we estimated the incidence of childhood epilepsy, based on treatment, clinical diagnosis or symptomatic presentations recorded in primary care clinical records, and evaluated how this has changed over time and by sociodemographic factors such as age, gender and social deprivation.
Methods
Setting
Approximately 98% of the UK population are registered with a general practitioner (GP) who provides universal primary care services, acts as a gatekeeper for access to secondary care and shares in the care of patients seen in secondary care.13
Data source
The Health Improvement Network (THIN) is a clinical database which prospectively captures data on prescriptions, diagnoses and symptoms in patients presenting to primary care.14 Prescription data are automatically recorded each time a GP issues a prescription and coded using the UK Prescription Pricing Authority and classified according to the British National Formulary.15 ,16 Medical events are recorded by the GP when considered significant and coded using the Read system.17 Data on contact with secondary care are entered from referrals and discharge letters. Demographic data on age, gender and social deprivation (see details below) are also available. THIN contains anonymised records of approximately 5% of the UK population and is broadly representative of UK general practice in terms of age, gender and social deprivation.18 ,19 THIN captures valid and complete primary care data.20–22
Participants and cohorts
Included in the study were children aged 14 years or less who were born during 1994–2008. All participants were registered at (or within 6 months of) birth at 1 of 411 THIN practices. The primary analytical cohort included all eligible children and was used to estimate cumulative incidence. These children were followed from birth until the end of 2008, transfer to another practice or death; whichever occurred first.
Within the larger cohort, we examined a subcohort of children who were between 0 and 7 years (under 8 years) in the period 2001–2008. These children were epilepsy-free at entry into the subcohort. By focussing on this period and age range, there wasere approximately the same proportion of children of each year of age in each calendar year.
Identifying children with epilepsy
We developed three indicators for epilepsy in children, based on records for AED prescription, epilepsy diagnoses or epilepsy symptoms. Code lists for these indicators were reviewed by a paediatric neurologist (RFC), a paediatrician (RG) and a GP (FK). The code lists are available upon request from the corresponding author (WHM).
The first and most specific indicator (hereafter, we refer to this as indicator 1) was a prescription for an AED licensed for use in children in the UK, which was repeated within a 4-month period. As primary care databases do not hold information on the usage of medicine, we chose repeat prescriptions as a marker for continued treatment. The AEDs considered were Carbamazepine, Ethosuximide, Gabapentin, Lamotrigine, Levetiracetam, Oxcarbazepine, Phenobarbital, Primidone, Phenytoin, Stiripentol, Topiramate, Valproate, Vigabatrin, Lacosamide, Rufinamide, Tiagabine, Valproic Acid, Cobazam, Clonazepam, Piracetam, Acetazolamide and Nitrazepam.23 Although AED prescriptions are often initiated in secondary care, all repeat prescriptions are issued in primary care for budgetary reasons.
The second indicator (hereafter indicator 2) captured all children with repeat AED prescriptions, plus additional children with diagnostic codes for epilepsy. GPs in the UK received no financial incentives to enter diagnostic codes for paediatric epilepsy and often use prescription data to indicate a diagnosis.
The third and most sensitive indicator (hereafter indicator 3) captured all children with AED prescriptions or an epilepsy diagnosis, plus children with symptoms of epilepsy. Children were considered to have epilepsy symptoms if they had codes for non-febrile seizures on two or more occasions.
We are aware that AEDs are prescribed for other indications than epilepsy in adults, for example, mood disorders or migraines, and this might also be the case for children. Therefore, we validated the indicators by reviewing the full primary care medical records (which include all contact with primary care plus additional details of contact with secondary care via referrals and discharge letters) of a 10% random sample of all children identified using indicator 3. We looked for corroboratory evidence of epilepsy in their medical records and for clinical conditions which might rule out a definition of epilepsy (eg, repeat AED use for migraines). In this review, we used all data available, including records of referrals and hospital discharge information. This included diagnoses, symptoms and information from secondary care (when available) on epilepsy and indications for AEDs.
Statistical analysis
We defined the incident date of epilepsy as the earliest date of AED prescription, epilepsy diagnosis or seizure. Cumulative incidence was estimated in the full cohort of children using the Kaplan-Meier (KM) failure curves. To compare with previous studies, cumulative incidence was measured in children aged 1 and 5 years. The effect of year-of-birth was assessed by plotting KM failure curves for children born in 3-year birth groups. The log-rank test for trend was used to test the equality of failure function estimates between the 3-year birth groups.
The annual incidence of recorded epilepsy was calculated using the subcohort of children aged between 0 and 7 years in the period 2001–2008. Rates were calculated by calendar year, age, gender and Townsend quintile using person-years-at-risk (PYAR) as the denominator. Townsend score is a measure of social deprivation based on owner's occupation, car ownership, overcrowding and unemployment.24 In THIN, Townsend scores are derived from 2001 census data that have been linked to patient post codes and are thus area-based measures. Townsend scores in THIN are provided to researchers in quintiles. Incidence rate ratios (IRR) were calculated for each indicator using Poisson regression models for linear trend, with adjustment for clustering by practice. Age was included as a binary variable (<1 and 1–7 years) to reflect different incidence rates and aetiologies in infants and older children.6
All data management and analyses were performed using Stata SE V.11 (StataCorp, College Station, Texas, USA).
Ethics
Epidemiological research using THIN data has ethical approval from the South East Multi-Centre Research Ethics Committee (Protocol Number 03/01/073). Additional approval for this study was received from the THIN scientific review committee.
Results
Cumulative incidence
The full cohort included 344 718 children aged 0–14 years with 1 447 760 person-years of follow-up between 1994 and 2008. For the individual children, the mean follow-up was 4.2 years.
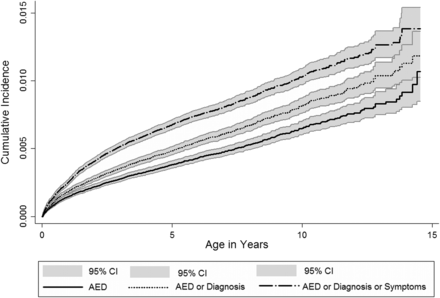
By the age of 1 year, the cumulative incidence of epilepsy was 0.15% (95% CI 0.13% to 0.16%) based on indicator 1 and 0.25% (95%CI 0.23% to 0.27%) based on indicator 3 (figure 1). Cumulative incidence at age 5 years was 0.38% (95% CI 0.36% to 0.41%) for indicator 1 and 0.68% (95% CI 0.64% to 0.71) for indicator 3.
Cumulative incidence of recorded epilepsy in children. Cumulative incidence of epilepsy in children aged 0–14 years between 1994 and 2008—using all three epilepsy indicators.
A significant difference (log-rank test for trend, p<0.001, χ2=104.0) in the cumulative incidence of epilepsy was observed among the 3-year birth groups. This is illustrated for the most sensitive indicator (3) in figure 2 where by age 5 years, 1.00% (95% CI 0.86% to 1.16%) of children born between 1994 and 1996 had epilepsy compared to 0.53% (95% CI 0.47% to 0.59%) of children born between 2003 and 2005. Children born in 2006–2008 did not reach age 5 years; however, the cumulative incidence trends in this group mirrored those of children born in 2003–2005. The observed decline (using indicator 3) represents a decline of 47%. Less-steep declines were observed using the more specific indicators (1 and 2). The decline in cumulative incidence at age 5 years among children born between 1994–1996 and 2003–2005 was 33% for the most specific indicator (1).
Cumulative incidence of recorded epilepsy in children by year-of-birth. Cumulative incidence of epilepsy in children aged 0–14 years between 1994 and 2008 by year-of-birth—using indicator 3 (antiepileptic drug prescriptions or an epilepsy diagnosis or epilepsy symptoms).
Annual incidence
The annual incidence of recorded epilepsy was estimated using a subcohort of 329 823 children aged 0–7 years with 1 045 629 person-years follow-up between 2001 and 2008. Rates are shown in table 1 and online supplementary tables S1 and S2.
Annual incidence of newly recorded epilepsy in children aged 0–7 years between 2001 and 2008 by gender, age and deprivation quintile—using indicator 3 (repeat antiepileptic drug prescriptions or an epilepsy diagnosis, or epilepsy symptoms)
Using the most sensitive indicator (3), the annual incidence was 116 (95% CI 110 to 123) per 100 000 PYAR. Annual incidence was 58% lower in those aged between 1 and 7 years than in those aged <1 year (1–7 years vs <1 year: IRR 0.42; 95% CI 0.37 to 0.47), and 17% lower in females than in males (females vs males: IRR 0.83; 95% CI 0.75 to 0.92). There was some variation in the incidence of epilepsy by the Townsend deprivation quintile (table 1). The incidence was lowest in the least deprived quintile, and relatively higher in the more deprived quintiles (3–5). However, there was no linear trend with increasing deprivation.
The annual incidence for children aged 0–7 years was 89.2 (95% CI 83.6 to 95.1) per 100 000 PYAR measured using indicator 2, and 70.6 (95% CI 65.6 to 75.9) per 100 000 PYAR measured using indicator 1. The age, gender and deprivation trends for these indicators were similar to trends for the most sensitive indicator (3).
The decline each year in the annual incidence between 2001 and 2008, after adjusting for age, gender and deprivation, was 4% (IRR 0.96; 95% CI 0.92 to 0.99) for indicator 1, 6% (IRR 0.94; 95% CI 0.91 to 0.97) for indicator 2 and 9% (IRR 0.91; 95% CI 0.89 to 0.94) for indicator 3 (figure 3). Although the annual incidence rate was higher for children aged <1 year than for children aged 1–7 years, the decline between 2001 and 2008 was similar in both age groups (see online supplementary figure S1). It should also be noted that the annual incidence appears to stabilise from around 2005/2006.
Annual incidence of recorded epilepsy in children. Annual incidence of epilepsy in children aged 0–7 years in the UK between 2001 and 2008—using all three epilepsy indicators.
Validation
Of the random sample of 183 children with epilepsy (indicator 3) whose medical records we examined in full, corroboratory evidence of epilepsy (eg, symptoms and diagnoses) was identified in 92%. The 8% without corroboratory evidence were receiving repeat prescriptions for AEDs but had no evidence to suggest that they were receiving AEDs for conditions other than epilepsy (eg, none had records for alternative indications such as migraine, psychiatric disorder, chronic pain or neonatal alcohol withdrawal syndrome).
Discussion
Both the cumulative and annual incidence of recorded epilepsy declined over time. By age 5 years, cumulative incidence using our most sensitive indicator (3) for epilepsy was 1.00% (95% CI 0.86% to 1.16%) for children born between 1994 and 1996 compared with 0.53% (95% CI 0.47% to 0.59%) for children born between 2003 and 2005. The decline in cumulative incidence was less for the more specific indicators (1 and 2). The annual incidence of recorded epilepsy declined between 4% and 9% per year, with the steepest decline seen for children with the most sensitive indicator (3).
Our estimates of cumulative incidence of recorded epilepsy at age 1 year (0.15–0.22%) and at age 5 years (0.38–0.68%) were similar to other population-based studies from Denmark,10 Iceland25 and the USA.26 Our estimates of annual incidence (71–116/100 000 PYAR) are also comparable with rates from industrialised countries (35–150/100 000 children).27 Comparison with previous UK studies is difficult due to variations in case definitions and populations studied. Earlier studies focused on AED use (defined as one or more prescriptions)12 or epilepsy in a small population (Tonbridge).2 Our study is the largest UK study to date that focuses exclusively on epilepsy treatment and management (including diagnosis) in young children.
We observed a near halving of the cumulative incidence of recorded epilepsy at age 5 years for children born between 1994–1996 and 2003–2005 and found a significant decline in the annual incidence of recorded epilepsy of 4–9% per annum between 2001 and 2008. Recent longitudinal studies with strict case definitions from Finland and Denmark also demonstrate evidence of a recent, but smaller, decline in the incidence of epilepsy.10 ,11 Our findings are supportive of a continuation of the decreasing trend in the incidence of epilepsy reported in earlier studies from the USA,26 ,28 Sweden29 ,30 and England.2 ,12 Only one recent study found no evidence of a decline,31 though a decreased incidence in children could have been masked by an increase in the elderly as the authors did not separately analyse trends for different age groups.26 These studies are summarised in table 2.
Studies reporting temporal trends in the incidence of epilepsy in children*
The reasons for the decline in paediatric epilepsy recorded in primary care are unclear. A shift to later diagnosis can be ruled out due to our observation of a parallel decline in children <1 and 1–7 years of age. Increases in diagnostic accuracy9 ,32 due to greater involvement of specialist services and advancements in diagnostic technology (leading to fewer false-positive epilepsy diagnoses) may partly explain the decline. A study in the early 1990s found that up to 20% of children with epilepsy were misdiagnosed, suggesting that a decline of up to 20% could have resulted from improved diagnosis.9
National guidelines released in 2004 focused on improved epilepsy diagnosis7 ,8 and could have had an impact on the incidence of epilepsy recorded in primary care. Indeed, steeper declines in recorded incidence were observed with the more sensitive indicators suggesting that policy changes may have been effective in reducing misdiagnosis of epilepsy. However, other factors may have contributed, as the steepest decline in incidence occurred prior to 2004. For example, clinical thresholds for diagnosis or treatment may have increased following awareness of the harms of treatment,33 or increasing reluctance by GPs to label a child with epilepsy when the diagnosis is uncertain. Additional changes in treatment practices may have played a role. For example, during the study period, treatment has ceased to be recommended for benign epilepsy syndromes and paroxysmal movement disorders, although these conditions were formerly treated as a form of epilepsy.
A further explanation is that changes in environmental exposure to epilepsy risk factors contributed to the observed decline in recorded epilepsy. Up to 22% of children with central nervous system (CNS) infections34 and 11% of children with traumatic brain injuries35 develop epilepsy. Rates for central nervous system infections fell dramatically in the UK following the introduction of meningitis vaccines in 1992, 1999 and 2006.36 ,37 Similarly, rates of hospital admission for intracranial head injuries in infants have been declining since 1997 (Ruth Gilbert, personal communication). Although it is unlikely that any single environmental factor could have contributed to the decline in the incidence of epilepsy, these factors, in combination with changes in prescribing practices and more specialist diagnostic services, may together account for the temporal trends we observed.
Trends in age6 and gender38 ,39 were largely similar to those of previous studies; however, we did not find a linear relationship between deprivation and epilepsy recording, which has been observed in studies among adults.4 ,40 While we found that those from the least deprived quintile of Townsend scores had the lowest incidence, it was those from the middle quintiles who had the highest incidence. Future studies may examine these patterns in more detail.
Limitations
Primary care data are recorded by GPs in order to help them care for patients. For this reason, primary care databases are subject to incomplete recording and misclassification.
We addressed problems of incomplete recording by using a range of indicators to identify children with epilepsy based on prescriptions, diagnoses and symptoms. The comparability of our incidence estimates with those from other countries provides indirect validation of our indicators. In addition, it is unlikely that an epileptic child will have no signs of epilepsy in their primary care record as GPs play an integral role in managing the health of epileptic children in the UK. In addition, the recording of epilepsy in primary care is likely to be accurate as the diagnoses recorded in primary care records are made by specialists in secondary care (not GPs). The diagnoses are then reported from the specialists in secondary care to the GP who enters the diagnosis into the medical records.
To assess misclassification, we examined medical records for a 10% random sample of (183) children identified with epilepsy (using indicator 3) and found no evidence of misclassification among these children. However, to rule out misclassification as a result of AED use for conditions other than epilepsy, we investigated a larger sample of children receiving repeat AED prescriptions. This sample included all children in the subcohort identified using indicator 1. We searched for diagnoses of other relevant indications (eg, bipolar disorder, attention deficit hyperactivity disorder and migraines), which were likely to be comorbid. Only 2 (<1%) of the 772 children in the larger sample had codes for other relevant indications. The use of AEDs in the UK for conditions other than epilepsy is unlikely as such usage is not inline with national guidelines.23 ,41–43
Our cohort was likely to be representative of children in the UK;18 ,19 however, by using a cohort where entry was restricted to birth, we may have underestimated annual and cumulative incidence. This is because children with neurological disability are difficult to follow up on,44 and one might, therefore, expect a higher attrition rate among children with epilepsy. This could lead to a situation where children with epilepsy leave the cohort earlier, perhaps before they receive treatment and before a diagnosis or symptoms are recorded in their medical record. In our study, children with epilepsy had on average 2.3 years more follow-up than children without epilepsy, suggesting that children with epilepsy were not leaving the cohort earlier than children without epilepsy (as might have been expected based on previous research on follow-up of children with neurological disabilities44) and we did not underestimate incidence. In any case, this bias would have no impact on the observed temporal trends.
Conclusions
We observed a decline in the cumulative and annual incidence of recorded epilepsy in UK primary care. In 2008, compared to 1994, GPs recorded treating fewer children for epilepsy, receiving fewer confirmatory diagnoses from secondary care for children with suspected epilepsy and caring for fewer children with unprovoked seizures. Part, but not all, of the observed decline in incidence is likely to be explained by an improved specificity in epilepsy diagnosis as shown by steeper declines over time with the more sensitive indicators (2 and 3). Other factors possibly contributing to the decline in incidence of epilepsy in this age group include fewer conditions treated as epilepsy and changes in risk factors within the population.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online figure
- Data supplement 2 - Online tables
Footnotes
-
Contributors WHM, IP and RG conceived and planned the study. WHM analysed and drafted the report with inputs from IP and RG. RFC commented on the interpretation of the results, and FK contributed to the design of the study. All the authors helped to draft the report. RG is the guarantor.
-
Funding This work was supported and undertaken at the Great Ormond Street Hospital (GOSH)/UCL Institute of Child Health (ICH), which received a proportion of funding from the Department of Health's National Institute of Health Research (NIHR) Biomedical Research Centres funding scheme. The ICH Centre for Paediatric Epidemiology and Biostatistics (CPEB) also benefited from funding support from the Medical Research Council (MRC) in its capacity as the MRC Centre of Epidemiology for Child Health. WHM's time was funded by a studentship grant from the MRC. RFC was funded by a NIHR funded Walport Academic Clinical Lectureship. IP received funding from the MRC (grant code G0601726).
-
Competing interests None..
-
Ethics approval UK MREC and THIN Scientific Review Committee.
-
Provenance and peer review Not commissioned; externally peer reviewed.