Article Text
Abstract
Objective To explore the effect of research ethics, governance and consent requirements and recent reforms on UK-wide follow-up of children with congenital heart defects (CHD).
Design Prospective cohort study.
Setting UK National Health Service.
Patients 3897 children with CHD requiring intervention, or resulting in death, before they were 1-year-old (1993–1995).
Main outcomes Impact on study protocol, timeliness and findings of a multicentre study of survival and quality of life.
Results The peer-reviewed study protocol was altered to accommodate ethics committee stipulations that researchers should not approach families directly with a request to participate and that the general practitioner's (GP) permission be sought before the local clinician could do so. Individual consent was required to confirm the vital status of participants and for future tracing of public death registrations. Local study registration took a median of 40 weeks (IQR 25–57). 180 (24%) of 739 surviving children (five centres) could not be contacted because their GP was untraceable (32), had changed (128) or considered contact inappropriate (20). Invitations could not be sent to 31% from the most deprived compared with 17% from the least deprived areas.
Conclusions Decision making concerning childhood interventions should be influenced by evidence on long-term outcomes. However, current UK research regulations hinder follow-up in multicentre studies. Stipulations preventing researchers contacting families directly with research invitations appear disproportionate to the risks, impede equitable access to research opportunities and introduce bias. The requirement for an individual's consent to confirm whether they are alive and monitor survival precludes effective long-term follow-up.
Statistics from Altmetric.com
Introduction
It is now 10 years since the Bristol inquiry addressed concerns about the ‘management of the care of children receiving complex cardiac surgical services at the Bristol Royal Infirmary between 1984 and 1995 [and made recommendations] to secure high-quality care across the NHS’.1 The public inquiry lasted 3 years, taking evidence from 577 witnesses, including over 200 parents. Expert evidence emphasised the lack of systematic data on long-term outcomes relevant to families and highlighted how difficult it is to acquire such evidence (box 1).
Box 1 Bristol Royal Infirmary inquiry expert comments on current knowledge of long-term outcomes of congenital heart defects
“Longer-term outcomes, such as deterioration in functional and neurological status, the need for re-intervention [and] late deaths were described [in the literature but] the quality and detail of reporting of such outcomes was inconsistent. [Assessment of children] early after surgery has the disadvantage that the findings may have limited predictive validity for understanding how the deficits will impact on a child's prospects for future education and independence. Research is needed both to document the range of late problems postoperative children experience and to understand how and when best to inform parents about these.”39
What is already known on this topic
▶ Research on the longer-term outcome for children with congenital heart defects is lacking.
▶ Research ethics and governance processes can pose disproportionate administrative and resource burdens on population-based studies.
▶ Requirements for clinician consent to trace or contact potential research participants or for individual consent to access their data can lead to selection bias.
What this study adds
▶ Ethics review and consent requirements extended a multicentre follow-up study by more than 1 year and jeopardised future monitoring of survival.
▶ Impractical requirements for written consent to ‘flagging’ for public death registrations led to bias and social exclusion.
▶ Current requirements to obtain ‘consent for consent’ pose disproportionate obstacles to participation in research.
Since the final report of the inquiry, new systems for monitoring outcomes after cardiac surgery have been developed, such as the Central Cardiac Audit Database. While an advance, this database focuses on survival after individual procedures rather than outcome for individual children. Although it includes survival at 1 year after surgery, this was unknown for around one fifth of children undergoing an arterial switch operation between 2000 and 2007.2 There is no provision for any quality of life component to outcome evaluation. A complementary approach is to prospectively follow historically identified registers or cohorts. This avoids re-collection of data,3 although there are practical and ethical difficulties, for example in ascertaining deaths and changes of address, or collecting identifiers to link datasets and safeguard against double-counting.4
We established the UK Collaborative Study of Congenital Heart Defects (UKCSCHD) to document longer-term outcomes, specifically survival and quality of life at 10–14 years of age of a nationally representative cohort of 3897 children with serious congenital heart defects (CHD) originally notified to a UK-wide study evaluating fetal diagnosis.5
In negotiating ethics and governance approvals, we addressed:
the use of patient identifiable data collected in the past before the Data Protection Act 1998
contact with families in the present, with the exception of those whose children had died
future monitoring of survival using public death registrations.
Using the UKCSCHD as a case study, we document the impact of research ethics and governance systems on the study protocol, conduct, timeliness and potential for bias, and discuss the proportionality of regulation to the risks inherent in the research. We reflect on the potential impact of recent initiatives by the UK Clinical Research Collaboration (UKCRC)6 and affiliated organisations, the NHS Constitution7 and Care Record Guarantee,8 and the Data Sharing Review9 (box 2) on the burden of regulation.
Box 2 The Data Sharing Review: conclusions and recommendations
Main conclusions
▶ There is a lack of transparency and accountability in the way organisations deal with personal information.
▶ There is confusion surrounding the Data Protection Act, particularly the way it interacts with other strands of law.
▶ Greater use could be made of the ability to share personal data safely, particularly in the field of research and statistical analysis.
▶ The information commissioner needs more effective powers and the resources to allow him to use them properly.
Recommendations specifically relating to health research
▶ Recommendation 15: We recommend that ‘safe havens’ are developed as an environment for population-based research and statistical analysis in which the risk of identifying individuals is minimised; furthermore, we recommend that a system of accrediting researchers to work within those safe havens is established.
▶ Recommendation 16: We recommend that government departments and others wishing to develop, share and hold datasets for research and statistical purposes should work with academic and other partners to set up safe havens.
▶ Recommendation 17: We recommend that the NHS should develop a system to allow approved researchers (bound by the same duty of confidentiality as the clinical team providing care) to work with healthcare providers to identify potential patients, who may then be approached to take part in clinical studies for which consent is needed.
Methods
The initial study protocol (figure 1) was peer reviewed and funded by the British Heart Foundation in 2003, approved by cardiologists from all 17 specialist centres contributing patients to the original cohort and subsequently registered with the NHS Trust Research and Development (R&D) Directorate of the central research team. It comprised a hospital records review of perioperative and early life factors for all children co-ordinated by the central research team but involving data collectors in each centre. The initial protocol proposed checking public death registrations to identify deaths before the central research team approached families. Participating parents of surviving children and the children themselves were then invited to contribute information about current health and quality of life, by questionnaire. Families were asked to approach ‘controls’ from among classroom peers to match the ‘cases’.
Initial study protocol.
Preliminary review by the research ethics committee corresponding to the central research team stipulated that families be contacted through local cardiologists only, as the historical cohort had not required parental consent. An application for ethics review, including written support from paediatric cardiologists in all specialist centres, was submitted to Trent Multi-centre Research Ethics Committee (Trent MREC).
We applied to the Office for National Statistics (ONS) to trace deaths through public death registrations and to ‘flag’ cohort survivors for future deaths. After 2002, the ONS application process included asking researchers to seek individual consent to flagging from all living members of a cohort (Patient Information Advisory Group (PIAG) reference: 4-07(h)/2002).10
We document the registration process after MREC approval for 15 of the 17 participating centres; the study had already been registered during ethics review at the study co-ordinating centre and detailed timings for each stage are unavailable for one centre.
We report the proportion of affected patients whose families could not be sent a questionnaire, the proportion not returning a questionnaire and the variation by area deprivation score based on current postcode. Postcodes were matched to the 2004 Index of Multiple Deprivation score using GeoConvert (V May 2006 version; http://www.geoconvert.mimas.ac.uk/) and summarised as quintiles.
Results
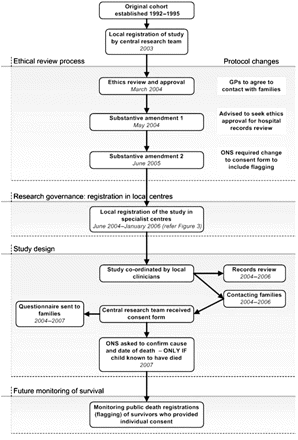
Changes to the initial study protocol required as a consequence of ethics review, local R&D registration and the ONS flagging application are summarised in figure 2.
Final study protocol influenced by research governance systems. GPs, general practitioners; ONS, Office for National Statistics.
Ethics review process
The ethics committee approved the revised study protocol in May 2004 but imposed two conditions: first, that the local cardiologist write to the general practitioner (GP) of each surviving child to ask if there was any reason (such as terminal illness or family distress) that precluded contact before writing to the family. There was no stipulation to approach the GPs of ‘control’ families. Second, the central research team was not to retain the names and addresses of cohort children originally notified to the principal investigator of the fetal diagnosis study (CB). These were to be held by the local cardiologist who would then be responsible for contacting families. The central research team was only to receive contact details directly from families agreeing to complete the questionnaire. These stipulations shifted a significant proportion of the administrative burden from central to local level.
At this point, the R&D Directorate at the central research team's institution reconsidered their registration of the study and advised that the hospital records review could not in fact be registered as an audit as it covered several centres, thus necessitating ethics approval through a substantive amendment. Initial ethics approval took 40 days and the amendment was approved in July 2004 after a further 54 days.
Research governance: NHS R&D registration
Although local cardiologists were not designated researchers, as a consequence of the above amendments they were required as ‘responsible clinicians’ to initiate registration of the project locally, manage the review of local hospital records and contact families. Although supported by the central research team, local registration of the study took much longer to complete than anticipated; most cardiologists had limited time to allocate to research and since agreeing to participate, some had retired, moved overseas or changed centre and so new ‘responsible clinicians’ had to be identified.
Local cardiologists took a median of 20 weeks (IQR 15–28 weeks) to initiate contact with their R&D office. Once notified of the study, despite peer review and MREC approval, R&D offices made a total of 30 requests (including six by one centre) for additional documents before accepting the final submission. The additional documentation required was extremely variable (box 3), although all centres requested the ethics approval letter and most requested completion of a local registration form. Two centres insisted on external peer review despite being provided with evidence of peer review from the British Heart Foundation and one centre required a further full local ethics review. The latter required that a new application form be completed: this took 20 weeks to finalise with the local cardiologist and 7 weeks to be approved. One centre mislaid the original paperwork and, in another, documents were irreparably damaged during flooding and had to be resubmitted. Median time from initial contact to complete submission of all requested documents was 6 weeks (IQR 2–13 weeks) and once all documents were submitted, median time to final registration was 8 weeks (IQR 7–13 weeks).
Box 3 Requests for additional information (n) made by NHS Trust Research and Development offices
Additional reviews
▶ External peer review (2), despite evidence of peer review by the British Heart Foundation
▶ Local ethics review (1)
Additional documents
▶ Copies of ethics application supporting documentation (13), for example, patient information and consent forms
▶ Completed electronic ethics application form (3)
▶ Letter of sponsorship (2)
▶ ‘Undertaking of responsibility’ by the principal investigator (3)
▶ Principal investigator's CV (1)
▶ Confirmation of good clinical practice training – usually only required for clinical trials (1)
▶ Proof of funding (1)
▶ Completion of local hospital trust risk assessment forms (1)
Across all centres, median time from ethics approval to full local registration, permitting commencement of data collection, was 40 weeks (IQR 25–57 weeks). In one third of centres local registration took over 1 year from the date of ethics approval (figure 3). In addition, seven honorary contracts had to be negotiated before the central research team were allowed to collect data in four hospitals.
Time from ethics approval to full local registration of study with the research and development (R&D) department.
Future monitoring of survival
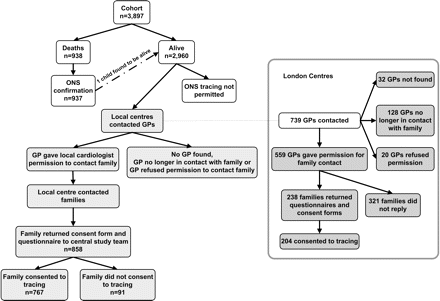
ONS agreed to confirm deaths through public registrations only if a child was known by their local cardiologist to have died. ONS declined to verify deaths in the entire cohort using public registrations without explicit individual consent, although the ethics approval even to approach families was contingent on certainty that the index child was still alive. Subsequently, deaths identified by clinicians were verified from public death registrations; one child presumed dead was found to be alive. We were unable, due to ONS requirements, to confirm that children presumed alive by clinicians were indeed alive (figure 4).
Flowchart describing the process of contacting families via GPs and clinicians. GP, general practitioner.
ONS also required that a specific question be added to the consent form to ask families to agree to future monitoring, or flagging, of their child's survival. This required a second substantive amendment from the ethics committee in June 2005 (figure 2). Forms consenting to receive a questionnaire were returned by 858 (29%) of 2960 families with surviving children who were approached, of whom 767 (89% of 858) consented to flagging, amounting to 26% of all surviving cohort children. Children living in more deprived areas were less likely to respond to the invitation to participate: fewer than 20% of those living in the most deprived quintile responded compared with 40% in the least deprived quintile (figure 5; χ2 test for trend=75.8, p<0.001).
Responses of parents to the invitation to take part in the study and in flagging (based on 2195 English and Welsh residents).
Barriers to contacting families were evaluated in greater detail in 739 surviving children (25% of all those surviving) whose invitation to participate was sent by the central research team acting on behalf of five London centres (figure 4). The GPs of 32 families (4%) could not be traced, GPs of 128 (17%) reported that the family had moved or changed GP, while GPs of 20 (3%) advised against family contact, citing specific reasons for 17, namely child disability (six), child or parent illness (six) or family dysfunction (five). Thus an invitation letter could be sent to 559 families, of whom 238 (43%) consented to the survey, with 204 (86% of those responding) to flagging. The invitation letter was returned in 27 (5%) cases as the child no longer lived at that address, while no reply was received for the remaining 294. Thirty-one per cent of children from the most deprived areas could not be sent an invitation letter compared with only 17% from the least deprived areas (OR 2.21, 95% CI 1.27 to 3.86). The most frequent reason for failure to contact a family was that they had moved; 27% of families within the most deprived quintile had moved compared with 11% within the least deprived (OR 0.34, 95% CI 0.18 to 0.63).
Discussion
Despite the recommendations of the Bristol inquiry, the survival and quality of life for children with CHD are not systematically monitored. Our national cohort study represented a valuable opportunity to address this meaningfully. Our experience of the UK regulatory framework illustrates how this changed the study protocol and methods for consent, extended data collection by over 1 year and impeded future follow-up of this unique cohort.
Although governance of healthcare research largely originated as a safeguard for participants in clinical drug trials,11 12 some regulatory procedures have developed as a reaction to nadirs in public trust,13 such as organ retention ‘scandals’. Considerable variation has been reported in the local interpretation of research regulations within the UK11 14,–,27 and the EuroSOCAP Project highlighted the wide ‘variety of legal provisions’ for data privacy across Europe.28 Guidance from the Council of International Organizations of Medical Sciences29 and the European Data Protection Directive (95/46/EC)30 both adhere to the principle of an individual's right to consent to the use of personal data but agree that this may be waived in certain circumstances, such as epidemiological research,29 provided that ‘there are secure safeguards of confidentiality’.28 In response to the increasing opportunities to link datasets offered by advances in information technology,31 concerns are now focusing on the security of personal medical data.32
The Data Sharing Review has presented an important set of new recommendations for change which respect the basic tenets of the Data Protection Act and are acknowledged within the NHS Care Record Guarantee.33 In our study, we asked cardiologists to identify and contact their own patients on our behalf and provided them with funding to do so. The additional requirement of the ethics committee, namely that cardiologists write to GPs for consent before contacting a family, resulted in both GPs and local cardiologists acting as ‘gatekeepers’ to the patient's receipt of invitations to take part in research. These ‘consent for consent’ requirements, in our view, posed disproportionate obstacles to the equitable opportunity to participate in research. If the responsible cardiologist had written directly to families on our behalf as originally envisaged, perhaps facilitated by the new personal demographics service,34 families whose GPs could not be traced or for whom hospitals held outdated GP contact details could also have been contacted. As all the children in this study remained under regular follow-up by their cardiologist, we would have hoped through their involvement to avoid contacting families who would be distressed by the invitation to participate. Crucially, a few GPs refused contact with families because a child had additional disabilities, thus selectively excluding them from the opportunity to decide whether they wished to contribute follow-up information. By contrast we were not required to approach GPs of age-matched controls. Moreover, there is increasing experience of direct approaches to patients by UK researchers with little evidence of harm and much potential benefit: for example, researchers in the UK Collaborative Trial of Ovarian Cancer Screening approached over 1.2 million postmenopausal women and recruited 202 638; only 32 complained about being contacted.35 Review and synthesis of the governance experience of this and similar studies would provide an evidentiary basis from which to explore alternative mechanisms for recruitment in large studies.
New legislation to support the recommendations of the Data Sharing Review would enable future access to personal medical information by ‘approved researchers’ within a secure data environment. It would also allow access to contact details so that the public can receive invitations to participate in research while respecting patient consent, confidentiality and individual privacy, as outlined in the NHS Care Record Guarantee.8 These steps would recognise the important contribution made by research to improving health services and shared decision making. Moreover, it would allow parents and children greater autonomy in decisions to take part in research and avoid imposing assumptions about appropriateness of research participation based on disability.
The duration and complexity of our study was increased by the need to complete multiple registrations, each with different requirements and interpretations and to negotiate several honorary contracts. The UKCRC and partners have recently introduced reforms to address the disproportionate obstacles presented by UK research regulation; these might have removed some of the difficulties that we encountered in our original study protocol. In addition, researchers are encouraged to anticipate these hurdles by a variety of innovations including the MRC Data and Tissues Toolkit36 and the National Research Ethics Service (NRES) Integrated Research Application System. NRES has developed a single application portal and a Research Passport, which removes the need for multiple honorary contracts, while the UKCRC Regulatory and Governance Advice Service and NRES quality assurance programme are improving the consistency of advice provided. Early evaluation of the success of these approaches in improving the timeliness of research initiation will be essential.
The ONS requirement for individual written consent to flagging was introduced in 2001; provision is made for approval to be sought under Section 251 of the National Health Service Act 2006 (formerly Section 60 of the Health and Social Care Act 200137) if this cannot be obtained. Previous researchers have reported the practical difficulties and potential for selection bias resulting from a requirement for individual written consent within a national clinical audit database.16 Our study provides evidence that this requirement leads to disproportionate exclusion of participants from more deprived areas, which may be related to factors such as lower literacy levels or increased mobility, making them more difficult to trace.38 Perversely, the requirement for consent prevented us from using death registrations to avoid contacting and potentially distressing families of children who had died. Responsibility for approving flagging applications transferred from ONS to the Patient Information Advisory Group (now the NIGB Ethics and Confidentiality Committee or ECC) in April 2008, allowing this group to determine when individual consent may not be practicable as, for example, in large population-based studies of survival. We now plan to seek approval to undertake flagging without consent under Section 251 of the NHS Act 2006.
In conclusion, it is evident that despite recent improvements, current UK research regulations and their interpretation continue to pose several obstacles to the long-term follow-up of children in multicentre studies. If clinicians are to take on the role of contacting patients and their families directly in order to invite their participation in research, more attention should be given to providing the resources needed for this to be undertaken. Furthermore, consideration should be given to the proportionality of the requirement to obtain an individual's consent to confirm whether they are alive and to monitor their future survival, as this precludes effective long-term follow-up to gather reliable information regarding outcomes for children with chronic conditions. Finally, current processes involved in obtaining ‘consent for consent’ whereby a clinician is given a gatekeeper function, need review as there is evidence that this may impede equitable access to research.
Acknowledgments
We are grateful to the UK paediatric cardiologists and members of the British Congenital Cardiac Association who have supported the UK Collaborative Study for Congenital Heart Defects and undertaken data collection and contacting families for the study. We are also grateful to Heartline (patient support group) for supporting this study.
References
Footnotes
-
Funding The UK Collaborative Study for Congenital Heart Defects is funded by a British Heart Foundation project grant (reference PG/02/065/13934). RLK was funded by an MRC special training fellowship in Health of the Public and Health Services Research (Fellowship Number G106/1083). This work was undertaken at GOSH/UCL Institute of Child Health which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme. The Centre for Paediatric Epidemiology and Biostatistics benefits from funding support from the Medical Research Council in its capacity as the MRC Centre of Epidemiology for Child Health. The funders had no role in the study design, data analysis and interpretation, writing or publication of this paper.
-
Competing interests CD is a representative member (for the Academy of Medical Sciences) of the National Information Governance Board (NIGB), a member of the Ethics and Confidentiality Committee representing NIGB, a member of the MRC Ethics Regulation and Public Involvement Committee and Strategy Board and the OSHR e-Health Board. She is writing in a personal capacity. All other authors declared that they have no conflicts of interest.
-
Ethics approval This study was conducted with the approval of the Trent MREC, reference no. 04/4/017.
-
Provenance and peer review Not commissioned; externally peer reviewed.