Published online Mar 28, 2020. doi: 10.3748/wjg.v26.i12.1352
Peer-review started: November 24, 2019
First decision: December 30, 2019
Revised: February 17, 2020
Accepted: February 28, 2020
Article in press: February 28, 2020
Published online: March 28, 2020
Pediatric living donor liver transplantation (LDLT) has become the gold standard for patients with end-stage liver disease. With improvements in organ preservation, immunosuppression, surgical and anesthesia techniques, the survival rates and long-term outcomes of patients after LDLT have significantly improved worldwide. However, data on anesthetic management and postoperative survival rate of pediatric LDLT in China are rare.
To review the status of pediatric LDLT in Shanghai and investigate the factors related to anesthetic management and survival rate in pediatric LDLT.
We conducted a retrospective observational study to investigate the status of pediatric LDLT in Shanghai by reviewing 544 records of patients who underwent pediatric LDLT since the first operation on October 21, 2006 until August 10, 2016 at Renji Hospital and Huashan Hospital.
The 30-d, 90-d, 1-year, and 2-year survival rates were 95.22%, 93.38%, 91.36%, and 89.34%, respectively. The 2-year patient survival rate after January 1, 2011 significantly improved compared with the previous period (74.47% vs 90.74%; hazard ratio: 2.92; 95% confidence interval (CI): 2.16–14.14; P = 0.0004). Median duration of mechanical ventilation in the intensive care unit (ICU) was 18 h [interquartile range (IQR), 15.25–20.25], median ICU length of stay was 6 d (IQR: 4.80–9.00), and median postoperative length of stay was 24 d (IQR: 18.00–34.00). Forty-seven (8.60%) of 544 patients did not receive red blood cell transfusion during the operation.
Pediatric end-stage liver disease (PELD) score, anesthesia duration, operation duration, intraoperative blood loss, and ICU length of stay were independent predictive factors of in-hospital patient survival. Pediatric end-stage liver disease score, operation duration, and ICU length of stay were independent predictive factors of 1-year and 3-year patient survival.
Core tip: The annual caseload of pediatric living donor liver transplantation has been growing rapidly and recipients have achieved excellent outcomes in Shanghai with 2-year patient survival rate of 89.34%. Moreover, pediatric end-stage liver disease score, anesthesia duration, operation duration, intraoperative blood loss, and intensive care unit length of stay were independent predictive factors of in-hospital patient survival. Pediatric end-stage liver disease score, operation duration, and intensive care unit length of stay were independent predictive factors of 1-year and 3-year patient survival.
- Citation: Pan ZY, Fan YC, Wang XQ, Chen LK, Zou QQ, Zhou T, Qiu BJ, Lu YF, Shen CH, Yu WF, Luo Y, Su DS. Pediatric living donor liver transplantation decade progress in Shanghai: Characteristics and risks factors of mortality. World J Gastroenterol 2020; 26(12): 1352-1364
- URL: https://www.wjgnet.com/1007-9327/full/v26/i12/1352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i12.1352
Since the first successful pediatric liver transplantation (LT) in 1967 by Starzl et al[1], LT has become the gold standard for patients with end-stage liver disease[2]. To overcome severe donor shortage, Bismuth and Houssin first described reduction of an adult liver to enable its transplantation into a pediatric recipient[3]. In 1989, successful pediatric living donor liver transplantation (LDLT) was performed by Strong et al[4], who transplanted the left lobe of a mother’s liver into her child. With improvements in organ preservation, immunosuppression, surgical and anesthetic techniques, the survival rates and long-term outcomes of patients after LDLT have markedly improved worldwide[5-8]. At present, LDLT is the most common form of LT in Asia[9,10].
The increasing use of grafts from living donors has enabled more children to survive with LT, and there has been a tremendous increase in pediatric LT in China[11,12] where the first LDLT was performed in 2001. However, data on anesthetic management and postoperative survival rate of pediatric LDLT in China are scarce. Therefore, we conducted a retrospective observational analysis of pediatric LDLT to review the status of LDLT in Shanghai and investigate factors related to anesthetic management and survival rate in pediatric LDLT.
We conducted this study in accordance with the tenets of the Declaration of Helsinki, and the Clinical Research Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University. The Clinical Research Ethics Committee of Huashan Hospital, Fudan University approved the protocol [Rj (2018) 218].
In Shanghai, two hospitals (Renji and Huashan) had performed pediatric LDLT by the end of 2016. We reviewed 559 records of all the pediatric LDLTs performed between October 21, 2006 and August 10, 2016 in Renji Hospital (School of Medicine, Shanghai Jiaotong University, n = 543) and in Huashan Hospital (Fudan University, n = 16), and set up a follow-up cut-off date of August 10, 2018. Data from 15 patients who were older than 12 years at the time of LT were excluded, and the final analysis comprised data from 544 children (Renji Hospital 530 LDLTs, Huashan Hospital 14 LDLTs).
Demographic data, including age, body weight, gender, diagnoses, preoperative PELD score, and history of Kasai procedure (KP) were extracted. Additionally, general donor information (age, donor liver weight, donor liver cold ischemia time, the relationship between donor and recipient) was collected. The etiology of transplantation was classified into three categories (cholestatic, metabolic, and others).
We documented the following intraoperative details: Anesthesia duration, surgery duration, intraoperative blood loss, donor liver weight, donor liver cold ischemia time, intraoperative packed red blood cell (PRBC) transfusions, total volume of liquid, transfusion threshold, and postoperative hemoglobin (Hb) level.
Additionally, we collected postoperative data including duration of mechanical ventilation (MV) in the intensive care unit (ICU), ICU length of stay, postoperative length of stay, PRBC administered in the ICU; 30-d, 90-d, 1-year, and 2-year survival rates were calculated. We defined patient survival as the time between transplantation and the end of follow-up or death. We calculated the annual caseload to describe the transplant trends from 2006 to 2016. Furthermore, we also performed uni- and multivariable survival analysis to determine the predominant factors affecting patient survival.
The suitable age for donors ranged from 18 to 55 years. Donors were evaluated using routine blood examinations, liver and kidney function examination, chest radiography, and electrocardiography. The quality, volume, and anatomy of the donor liver were carefully evaluated using computed tomography angiography.
Recipients underwent a series of laboratory and imaging examinations prior to surgery. The severity of illness was measured using the pediatric end-stage liver disease and Child–Pugh scores. Complete medical history, physical, radiological and laboratory examinations, and cardiac ultrasound were carefully evaluated by the anesthesiologist. Moreover, growth and nutritional assessments were also performed prior to surgery.
All recipients underwent routine anesthesia management: Patients fasted 6 h for solid food and 2–4 h for clear fluid before anesthesia. The operating table was equipped with a warming blanket. All patients were anesthetized using sevoflurane by mask or total intravenous anesthesia, maintained with sevoflurane, muscle relaxant and sufentanil intravenously via a pump. Patients were monitored by electrocardiography, continuous arterial blood pressure, continuous central venous pressure, and pulse oximetry. End-tidal CO2, body temperature, and urine output were also monitored. If necessary, dopamine or norepinephrine was infused through the portal vein to maintain hemodynamic stability.
All surgical procedures were performed by experts in the pediatric LT technique in the Department of Liver Surgery. Intraoperative real-time cholangiography was essential and an ultrasonic surgical aspirator was used in the donor operation, and the graft was implanted into the recipient’s abdominal cavity using the piggyback technique. All patients underwent Roux-en-Y hepaticojejunostomy for bile duct reconstruction[11].
Blood gas analysis was regularly performed to detect levels of Hb and other biochemical indices after induction of anesthesia, before the anhepatic phase, in the middle of the anhepatic phase, 5–10 min after the neohepatic phase, and at the end of surgery. The intraoperative PRBC transfusion was determined according to the amount of intraoperative blood loss, hemodynamic stability, and Hb level. No cell salvage or venovenous bypass was performed in these patients. Platelets were not regularly used as they were an independent risk factor for survival after LT[13].
Patients were sent to ICU after the operation, where blood gas analysis was performed immediately upon arrival, and at least twice/day thereafter. Similarly, liver function tests and the coagulation profile were monitored. Doppler ultrasound was performed at the end of surgery and every 24 h for the first 7 postoperative days to assess the patency of liver vessels.
We carried out statistical analyses using the IBM SPSS Statistics 23.0 (SPSS Inc., Armonk, NY, United States). The Kolmogorov–Smirnov test was carried out to obtain all distributions for normality. We expressed continuous variables as means ± standard deviation or medians with interquartile range (IQR: 25%–75%), and reported categorical variables as numbers (n) or proportions (%). We plotted the survival rates of recipients using Kaplan–Meier curves, and evaluated the differences in selected factors using the log-rank test.
To identify independent predictive factors of patients’ overall survival, univariable Cox regression analysis was used. Significant independent predictive factors (P < 0.05) were then included in the multivariable Cox proportional hazard regression analysis. Multivariable regression curves were also generated after multivariable Cox regression analyses. During the statistical analyses, 30-d survival analysis, 1-year survival analysis, 3-year survival analysis and overall survival analysis were all conducted to identify independent predictive factors of patients’ overall survival. To identify independent predictive factors of in-hospital patient survival, the χ2 test with the Yates correction or the Fisher’s exact test were used, as appropriate. All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
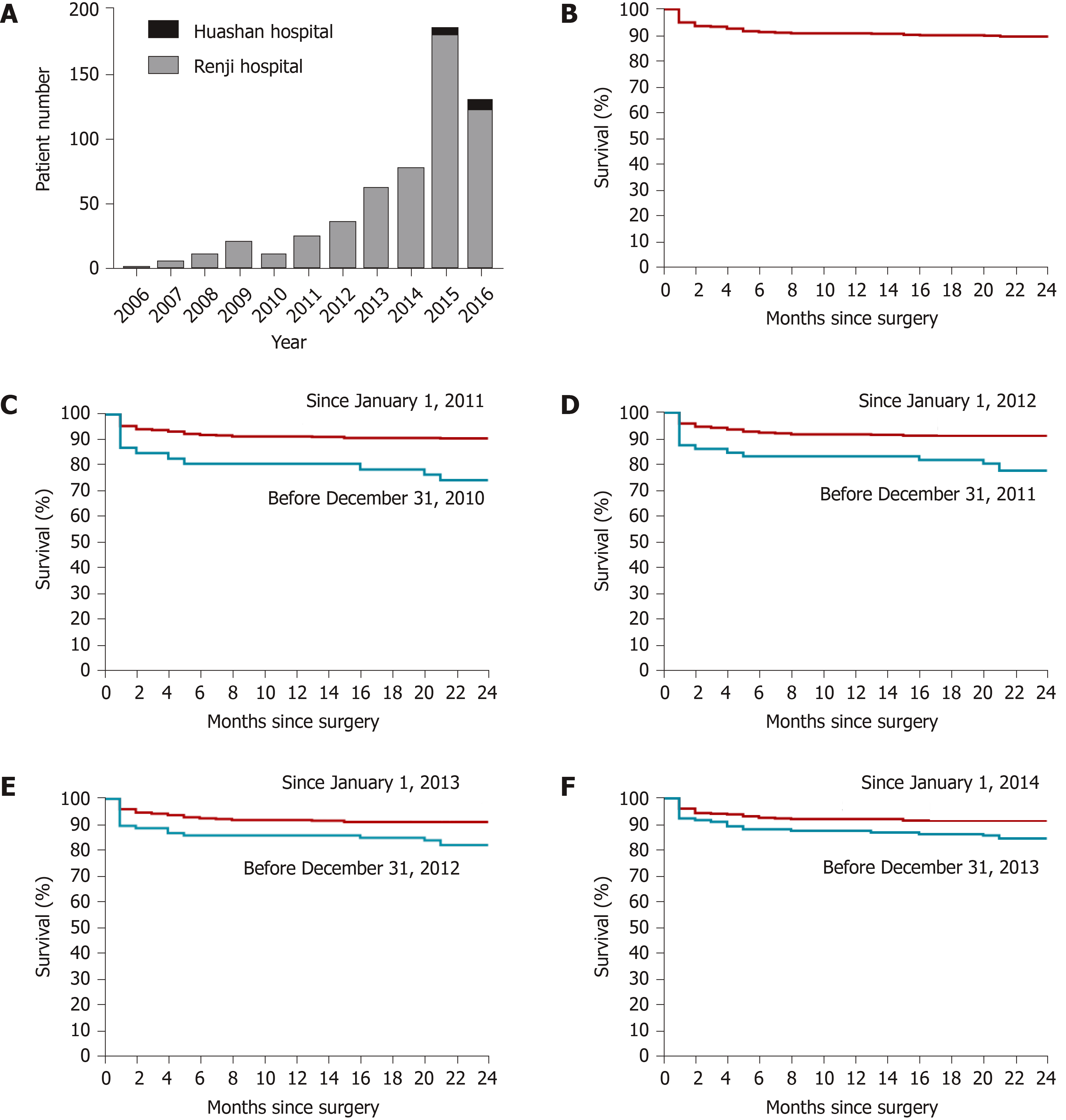
The first pediatric LDLT in Shanghai was performed at the Renji Hospital on October 21, 2006; and the first pediatric LDLT performed in Huashan Hospital was on September 3, 2014. As shown in Figure 1A, there was a rapid increase in pediatric LDLT cases from 2006 to 2016 with one case in 2006, which rose to 179 in 2015 (Renji Hospital 172, Huashan Hospital 5), and at the time of database lock on August 10, 2016, remained at 120 (Renji Hospital 112, Huashan Hospital 8).
After surgery, 518 (95.22%) of the 544 patients had survived at least 30 d, 508 (93.38%) at least 90 d, 497 (91.36%) at least one year, and 486 (89.34%) at least two year (Figure 1B). We found that the 2-year patient survival rate improved from 74.47% before 2011 to 90.74% after January 1, 2011 [hazard ratio (HR) = 2.92; 95% confidence interval (CI): 2.16–14.14; P = 0.0004] (Figure 1C). Similarly, the 2-year patient survival rate after January 1, 2012 had improved (91.10%) compared with the previous period (77.78%) (HR = 2.63; 95%CI: 1.85–8.67; P = 0.0005) (Figure 1D), the 2-year survival rate in 2013 (91.08%) had improved compared to the previous period (82.24%) (HR = 2.06; 95%CI: 1.29–4.77; P = 0.007) (Figure 1E), and the 2-year survival rate in 2014 (91.48%) had improved compared to the previous period (84.52%) (HR = 1.86; 95%CI: 1.15–3.67; P = 0.016) (Figure 1F).
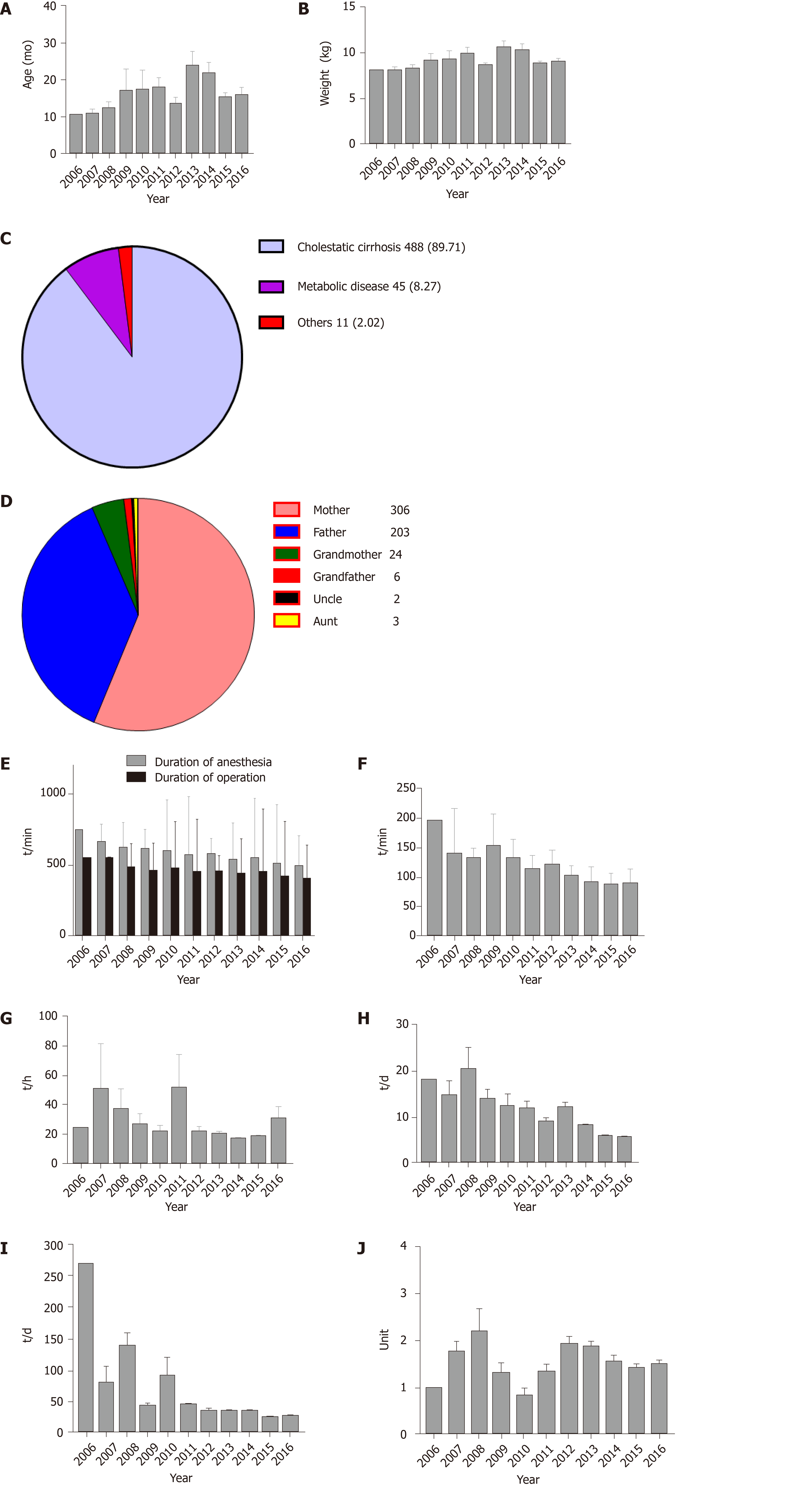
As shown in Figure 2A and B, the median recipient age was 8.20 mo (IQR: 7.00–13.95), and the median weight was 7.70 kg (IQR: 6.80–9.20). Cholestatic cirrhosis was the most common indication for LT in this study; 488 recipients (89.71%) were diagnosed as having cholestatic cirrhosis preoperatively (Figure 2C) and 216 (39.85%) of the 544 recipients had undergone KP prior to LT (Table 1).
| Variable | Value |
| Recipient variables (n = 544) | |
| Age (mo) | 8.20 (7.00-13.95) |
| Weight (kg) | 7.70 (6.80-9.20) |
| Male (%) | 257 (47.24) |
| PELD score | 17.00 (10.00-21.00) |
| Previous KP | 216 (39.85%) |
| Donor variables (n = 544) | |
| Donor age (yr) | 31.00 (27.00-36.00) |
| Donor liver weight (g) | 255.00 (225.00-290.00) |
| Donor liver cold ischemia time (min) | 65.50 (56.00-77.00) |
The 544 living donors examined in this study comprised 306 (56.3%) mothers, 203 (37.3%) fathers, 24 (4.4%) grandmothers, 6 (1.1%) grandfathers, 3 (0.6%) aunts, and 2 (0.4%) uncles (Figure 2D). The average donor age was 32.27 ± 7.24 years, the average donor liver weight was 261.95 ± 64.67 g, and the average donor liver cold ischemia time was 69.15 ± 26.14 min (Table 1).
The median anesthesia duration was 525 min (IQR: 476.50–587.50), and the median operation duration was 425 min (IQR: 384.00–480.00) (Figure 2E). The time period between anesthesia induction and surgery initiation showed a decreasing trend, which started at 194 min in 2006, and fell to 88.00 min (IQR: 77.50–98.50) in 2016 (Figure 2F).
The overall median ICU MV duration was 18 h (IQR: 15.2 5–20.25) (Figure 2G), the median ICU length of stay was 6 d (IQR: 4.80–9.00) (Figure 2H), and the median postoperative length of stay was 24 d (IQR: 18.00–34.00) (Figure 2I).
The median intraoperative blood loss was 200 mL (IQR: 100.00–250.00) and the median perioperative PRBC transfusion (intraoperative transfusion + ICU transfusion) was 1 unit (IQR: 1.00–2.00) (Figure 2J). The median blood transfusion threshold was 6 g/dL (IQR: 5.40–6.70). Of the 544 patients, 47 (8.60%) did not require PRBC transfusions during the operation, and 35 (6.40%) did not receive perioperative PRBC transfusions (Supplemental Table 1).
Univariable analysis was performed on 18 variables that may have an impact on the patient survival rate, including age (≤ 36 mo or > 36 mo), gender (male or female), recipient body weight (≤ 10 kg or > 10 kg), diagnosis (cholestatic cirrhosis, metabolic disease, or others), PELD score (≤ 2 or > 2), previous KP (yes or no), preoperative Hb level (≤ 8 g/dL or > 8 g/dL), anesthesia duration (≤ 600 min or > 600 min), operation duration (≤ 480 min or > 480 min), anhepatic phase (≤ 40 min or > 40 min), intraoperative blood loss (≤ 200 mL or > 200 mL), graft to recipient weight ratio (< 2.0%, 2.0%–4.0%, or ≥ 4.0%), cold ischemia (≤ 60 min or > 60 min), perioperative PRBC transfusion (≤ 1 U or > 1 U), perioperative blood transfusion (yes or no), ICU MV duration (≤ 24 h or > 24 h), ICU length of stay (≤ 9 d or > 9 d), and postoperative length of stay (≤ 30 d or > 30 d).
Of the 18 variables examined, PELD score, anesthesia duration, operation duration, intraoperative blood loss and ICU length of stay were independent predictive factors of in-hospital patient survival (P < 0.05) (Table 2). In addition, four of these factors were associated with 1-year patient survival, including PELD score (P = 0.029), preoperative Hb level (P = 0.034), operation duration (P = 0.016), and ICU length of stay (P = 0.003) (Supplemental Table 2). When these variables were fitted into the multivariable Cox regression model, three (PELD score, operation duration, and ICU length of stay) predicted 1-year patient survival (Table 3). For the 3-year patient survival, 3 of the 18 variables, including PELD score (P = 0.016), operation duration (P = 0.013), and ICU length of stay (P = 0.002) were independent predictive factors (Supplemental Table 3) in univariable analysis, and in the multivariable Cox regression model (Table 4).
| Independent predictive factor | Hospital death | P value | |
| Yes | No | ||
| Age (mo) | |||
| ≤ 36 | 35 | 440 | 0.847 |
| > 36 | 4 | 64 | |
| Gender | |||
| Male | 19 | 238 | 0.857 |
| Female | 20 | 266 | |
| Weight (kg) | |||
| ≤ 10 | 33 | 386 | 0.266 |
| > 10 | 6 | 116 | |
| Diagnosis | |||
| Cholestatic cirrhosis | 38 | 449 | 0.247 |
| Metabolic disease | 1 | 44 | |
| Others | 0 | 11 | |
| Previous KP | |||
| No | 25 | 301 | 0.611 |
| Yes | 14 | 201 | |
| PELD score | |||
| ≤ 2 | 36 | 372 | 0.0321 |
| > 2 | 3 | 118 | |
| Preoperative Hb (g/dL) | |||
| ≤ 8 | 15 | 121 | 0.058 |
| > 8 | 24 | 369 | |
| Anesthesia duration (min) | |||
| ≤ 600 | 26 | 408 | 0.0271 |
| > 600 | 13 | 94 | |
| Operation duration (min) | |||
| ≤ 480 | 22 | 388 | 0.0031 |
| > 480 | 17 | 114 | |
| Anhepatic phase (min) | |||
| ≤ 40 | 22 | 227 | 0.145 |
| > 40 | 16 | 270 | |
| Intraoperative blood loss (mL) | |||
| ≤ 200 | 23 | 375 | 0.0451 |
| > 200 | 15 | 123 | |
| GRWR (%) | |||
| 2 ≤ x ≤ 4 | 28 | 348 | 0.593 |
| < 2 or > 4 | 8 | 124 | |
| Cold ischemia time (min) | |||
| ≤ 60 | 17 | 178 | 0.241 |
| > 60 | 18 | 284 | |
| Perioperative RBC transfusion (U) | |||
| ≤ 1 | 22 | 320 | 0.369 |
| > 1 | 17 | 183 | |
| Perioperative blood transfusion (U) | |||
| Yes | 39 | 466 | 0.099 |
| No | 0 | 38 | |
| Postoperative length of stay (d) | |||
| ≤ 30 | 27 | 337 | 0.278 |
| > 30 | 8 | 156 | |
| ICU MV duration (h) | |||
| ≤ 24 | 20 | 370 | 0.199 |
| > 24 | 6 | 60 | |
| ICU length of stay (d) | |||
| ≤ 9 | 13 | 342 | 0.0001 |
| > 9 | 15 | 95 | |
| Independent predictive factor | HR | 95%CI | P value |
| ICU length of stay (d) | |||
| ≤ 9 | 1 | ||
| > 9 | 2.101 | 1.104-3.999 | 0.024 |
| ICU MV duration (h) | |||
| ≤ 8 | 1 | ||
| > 8 | 2.230 | 1.159-4.290 | 0.016 |
| PELD score | |||
| ≤ 2 | 1 | ||
| > 2 | 3.729 | 1.133-12.274 | 0.030 |
| Preoperative Hb (g/dL) | |||
| ≤ 8 | 1 | ||
| > 8 | 0.637 | 0.327-1.239 | 0.184 |
| Independent predictive factor | HR | 95%CI | P value |
| ICU length of stay (d) | |||
| ≤ 9 | 1 | ||
| > 9 | 1.966 | 1.122-3.446 | 0.018 |
| Length of surgery (h) | |||
| ≤ 8 | 1 | ||
| > 8 | 1.904 | 1.066-3.402 | 0.030 |
| PELD | |||
| ≤ 2 | 1 | ||
| > 2 | 3.659 | 1.303-10.274 | 0.014 |
Ever since the first successful LT was performed in mainland China in 1977, hundreds of centers in the region have followed suit. However, the development of pediatric LT has lagged behind that of adult LT due to social, cultural, and financial factors[11,12,14]. Renji Hospital is currently the largest transplant center for pediatric LT in China, and in close collaboration with the Shanghai Children’s Medical Center, aims to maximize benefit for children[11]. Pediatric LDLT has progressed immensely, and the procedure has become more frequent (1 in 2006, 179 in 2015, and 404 in 2017). Others have demonstrated a positive correlation between graft survival rates and the number of cases per center because as the transplant center gained technical experience with increased caseload, the incidence of surgical complications reduced, which improved patient and graft survival rates[11,12].
Pediatric LDLTs have better outcomes than split liver transplantation as LDLT conferred a 14.4% survival benefit in 3-year survival rate (82.1% vs 67.7%, respectively)[11]. Analyses of the collaborative transplant study database showed that long-term (5 years) graft survival was significantly better after LDLT (78.2%) than after deceased donor liver transplantation (71.4%)[15]. Grafts from living donors have potential advantages such as shortened wait times, optimal donor graft, and the ability to optimize the physical and psychological conditions of children with end-stage liver disease[12,16]. Kasahara et al[17] reported LDLT survival rates of 88.3%, 85.4%, 82.8%, and 79.6% for 1, 5, 10, and 20 years, respectively. Chen et al[18] reported a 5-year LDLT survival rate of 98% for patients with biliary atresia (BA), 94% for patients with hepatitis B virus cirrhosis, and 90% for patients with hepatocarcinoma. The survival rates observed in the Japanese LDLT series for BA were excellent, approaching 90.4%, 87.9%, 84.6%, and 79.9% for patients at 1, 5, 10, and 20 years post-LDLT, respectively[19]. In the United States, the overall 10-year actuarial graft and patient survival for LT in BA cases were 73% and 86%, respectively[20].
In the present study, we demonstrated that besides the increased annual caseload, postoperative outcomes also markedly improved in Shanghai—95.22% within the first 30 d, 93.38% within 90 d, 91.36% within 1 year, and 89.34% within 2 years. These survival rates are comparable to those in developed countries and may be attributed to financial support provided by social charities, improvements in surgical and anesthetic techniques, and the development of pre- and postoperative management strategies with feedback on long-term outcomes that significantly decreased the incidence of posttransplant complications.
In our survival analyses, PELD score, operation duration, and ICU length of stay were independent predictive factors of in-hospital patient survival as well as of 1-year and 3-year patient survival. A previous study showed that the PELD model accurately estimated the 90-d wait list mortality of pediatric LT candidates; however, the ability of the PELD score to predict postoperative mortality in pediatric patients was limited[21]. In contrast, we showed that a PELD score of > 2 was an independent risk factor of in-hospital, 1-year and 3-year patient survival. In our study, the median PELD score was 17.00, revealing that the preoperative condition of most children was critical. Furthermore, we identified that operation duration of > 480 min and ICU length of stay of > 9 d were risk factors.
The ICU management technique plays an important role in LT. Many studies showed that long-stay patients (LSPs) in pediatric ICUs have higher mortality rates than short-stay patients[22,23]. The mortality rates for LSPs and non-LSPs were 17.4% and 7.3%, respectively[23]. Furthermore, prolonged ICU length of stay is associated with increased hospital mortality and impaired patient and graft survival after LT[24]. Prolonged ICU length of stay may be due to the medical conditions of the patients, management-related progress between ICU and wards, and different medical care habits of different physicians. Whatever the reason, our data demonstrated that prolonged ICU length of stay is a predictive factor of long-term survival of patients. Even if prolongation of ICU stay is because patients are sick or because they have a complicated intraoperative course, ICU length of stay, as a result of multiple factors, predicts the patients’ outcome.
It would be interesting to determine whether ICU length of stay per se leads to poor outcomes. To address this, we performed propensity score matching adjustment to clarify the role of ICU stay in survival rate. We applied 1:1 nearest neighbor matching without replacement to ensure that conditional bias was minimized. We choose 0.1 as the caliper width. Propensity score, ICU length of stay, length of surgery, and PELD score were included in the multivariable Cox regression analysis. After propensity score matching adjustment, we found that ICU length of stay was still a predictive factor of patients’ 3-year survival (P = 0.046). These results indicate that, even for patients with similar conditions, longer ICU stay is detrimental for long-term survival and that a shorter ICU stay should be pursued for these patients in the future.
Our ICU management has made a lot of progress. A new immunosuppressive medicine, tacrolimus, has been in use in our clinic since 2010. This drug has been proved to have better effects and fewer adverse events[25]. In addition, clean laminar flow ICUs have been helpful in reducing postoperative infection since 2010.
Between 2006 and 2016, our anesthetic technique has also made progress. The use of ultrasound-guided central vein puncture since 2011 at Renji Hospital has vastly improved the speed and accuracy of puncture. Furthermore, the use of pulse-indicated continuous cardiac output since 2014 at Renji Hospital has offered a method for obtaining detailed information on hemodynamics, including continuous (pulse contour) cardiac output, cardiac preload, systemic vascular resistance, and extravascular lung water[26]. Thromboelastography has been used since 2016 at Renji Hospital to simultaneously assess coagulation factors, platelet function, clot strength, and fibrinolysis and can provide a complete picture of hemostasis[27].
We are aware of the limitations of our study. Its retrospective nature carries the usual limitations associated with these types of studies. Furthermore, we did not consider recipients’ intra- and postoperative complications and did not provide analyses on these data. Additionally, we did not analyze factors associated with mortality or whether the survival rate was related to the lower red blood cell (RBC) transfusion threshold. We propose to obtain these data from future prospective studies.
The anesthetic management of pediatric LDLT not only considers physiologic and metabolic changes that occur with end-stage liver disease but also involves an understanding of the peri- and postoperative management and factors affecting long-term outcomes. In this study, we reported survival rates of pediatric LDLT and its anesthetic management and trends in pediatric LDLT intervention in Shanghai, China. These results complement data on pediatric LDLT in China and will provide tools for improvement in the future.
In summary, the annual caseload of pediatric LDLT has been growing rapidly and recipients have achieved excellent outcomes in Shanghai with a 2-year patient survival rate of 89.34%. Of 544 patients, 47 (8.60%) did not require RBC transfusion intraoperatively, and 35 (6.40%) did not require any RBC transfusion perioperatively. The ICU and postoperative length of stay showed declining trends. Moreover, the PELD score, anesthesia duration, operation duration, intraoperative blood loss, and ICU length of stay were independent predictive factors of in-hospital patient survival. The PELD score, operation duration, and ICU length of stay were independent predictive factors of 1-year and 3-year patient survival.
Since the first successful pediatric living donor liver transplantation (LDLT) in 1989 by Strong, the procedure has become the gold standard for patients with end-stage liver disease. With improvements in organ preservation, immunosuppression, and surgical and anesthesia techniques, the survival rates and long-term outcomes of patients after LDLT have significantly improved worldwide. However, data on anesthetic management and postoperative survival rate of pediatric LDLT in mainland China are rare.
The purpose of this study was to provide information on the status of pediatric LDLT in Shanghai in recent years and to determine which of the perioperative management-related factors might impact survival rate in pediatric LDLT. These findings will help to optimize perioperative management in pediatric patients receiving LDLT in the future.
To summarize the status of pediatric LDLT in Shanghai in recent years and to investigate the impact of perioperative management-related factors on the survival rate in pediatric LDLT.
A retrospective observational study was conducted by reviewing 544 records of patients who underwent pediatric LDLT since the first operation on October 21, 2006 until August 10, 2016 at Renji Hospital and Huashan Hospital. Cox regression analysis was used to identify independent predictive factors of patients’ overall survival.
The 30-d, 90-d, 1-year, and 2-year survival rates were 95.22%, 93.38%, 91.36%, and 89.34%, respectively. The 2-year patient survival rate after January 1, 2011 significantly improved compared with the previous period (74.47% vs 90.74%; hazard ratio: 2.92; 95%CI: 2.16–14.14; P = 0.0004). Moreover, the pediatric end-stage liver disease (PELD) score, operation duration, and intensive care unit (ICU) length of stay were independent predictive factors of 1-year and 3-year patient survival.
In summary, our findings demonstrated that the annual caseload of pediatric LDLT has been growing rapidly and recipients have achieved excellent outcomes in Shanghai with a 2-year patient survival rate of 89.34%. The PELD score, operation duration, and ICU length of stay were independent predictive factors of 1-year and 3-year patient survival.
The present retrospective study demonstrates that the PELD score, operation duration, and ICU length of stay are independent predictive factors of in-hospital patient survival as well as of 1- and 3-year patient survival, and suggests that prospective trials exploring the effects of these factors on pediatric LDLT patient survival are warranted.
We would like to extend our gratitude to Dr Ping Wan, Prof Qiang Xia for their altruistic help in data collections.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Enosawa S, Hilmi I, Rauchfuss F, Srivastava M S-Editor: Zhou JJ L-Editor: Webster JR E-Editor: Liu MY
| 1. | Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Hakala TR, Rosenthal JT, Porter KA. Evolution of liver transplantation. Hepatology. 1982;2:614-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 590] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Feierman DE, Yudkowitz FS, Hojsak J, Emre S. Management of a cadaveric orthotopic liver transplantation in a pediatric patient with complex congenital heart disease. Paediatr Anaesth. 2006;16:669-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 594] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Nacoti M, Cazzaniga S, Lorusso F, Naldi L, Brambillasca P, Benigni A, Corno V, Colledan M, Bonanomi E, Vedovati S, Buoro S, Falanga A, Lussana F, Barbui T, Sonzogni V. The impact of perioperative transfusion of blood products on survival after pediatric liver transplantation. Pediatr Transplant. 2012;16:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Wagner C, Beebe DS, Carr RJ, Komanduri V, Humar A, Gruessner RW, Belani KG. Living related liver transplantation in infants and children: report of anesthetic care and early postoperative morbidity and mortality. J Clin Anesth. 2000;12:454-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Gurevich M, Guy-Viterbo V, Janssen M, Stephenne X, Smets F, Sokal E, Lefebvre C, Balligand JL, Pirotte T, Veyckemans F, Clapuyt P, Menten R, Dumitriu D, Danse E, Annet L, Clety SC, Detaille T, Latinne D, Sempoux C, Laterre PF, de Magnée C, Lerut J, Reding R. Living Donor Liver Transplantation in Children: Surgical and Immunological Results in 250 Recipients at Université Catholique de Louvain. Ann Surg. 2015;262:1141-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Song AT, Avelino-Silva VI, Pecora RA, Pugliese V, D'Albuquerque LA, Abdala E. Liver transplantation: fifty years of experience. World J Gastroenterol. 2014;20:5363-5374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 68] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Rela M, Reddy MS. Living donor liver transplant (LDLT) is the way forward in Asia. Hepatol Int. 2017;11:148-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Jawan B, Wang CH, Chen CL, Huang CJ, Cheng KW, Wu SC, Shih TH, Yang SC. Review of anesthesia in liver transplantation. Acta Anaesthesiol Taiwan. 2014;52:185-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Li QG, Wan P, Zhang JJ, Chen QM, Chen XS, Han LZ, Xia Q. Liver transplantation for biliary atresia: A single-center study from mainland China. World J Gastroenterol. 2015;21:9638-9647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wan P, Xu D, Zhang J, Li Q, Zhang M, Chen X, Luo Y, Shen C, Han L, Xia Q. Liver transplantation for biliary atresia: A nationwide investigation from 1996 to 2013 in mainland China. Pediatr Transplant. 2016;20:1051-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | de Sá Oliveira RR, Módolo MP, Mizubuti GB, Ho AMH, de Barros GAM, Muniz da Silva L, Braz LG, Módolo NSP, Day AG, Phelan R, Navarro E Lima LH, Ganem EM. Total Spinal Anesthesia Failure: Have You Assessed the Sensory Anesthesia in Sacral Dermatomes? Anesth Analg. 2017;124:1674-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Shen Z, He Y, Zheng S, Fan J. The current status of pediatric liver transplantation in Mainland China. Pediatr Transplant. 2010;14:575-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Hackl C, Schlitt HJ, Melter M, Knoppke B, Loss M. Current developments in pediatric liver transplantation. World J Hepatol. 2015;7:1509-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Firl DJ, Sasaki K, McVey J, Hupertz V, Radhakrishnan K, Fujiki M, Eghtesad B, Miller CM, Quintini C, Hashimoto K. Improved Survival Following Living Donor Liver Transplantation for Pediatric Acute Liver Failure: Analysis of 20 Years of US National Registry Data. Liver Transpl. 2019;25:1241-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kasahara M, Umeshita K, Inomata Y, Uemoto S; Japanese Liver Transplantation Society. Long-term outcomes of pediatric living donor liver transplantation in Japan: an analysis of more than 2200 cases listed in the registry of the Japanese Liver Transplantation Society. Am J Transplant. 2013;13:1830-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Chen CL, Concejero AM, Cheng YF. More than a quarter of a century of liver transplantation in Kaohsiung Chang Gung Memorial Hospital. Clin Transpl. 2011;213-221. [PubMed] [Cited in This Article: ] |
| 19. | Kasahara M, Umeshita K, Sakamoto S, Fukuda A, Furukawa H, Sakisaka S, Kobayashi E, Tanaka E, Inomata Y, Kawasaki S, Shimada M, Kokudo N, Egawa H, Ohdan H, Uemoto S; Japanese Liver Transplantation Society. Living donor liver transplantation for biliary atresia: An analysis of 2085 cases in the registry of the Japanese Liver Transplantation Society. Am J Transplant. 2018;18:659-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Harambat J, Ranchin B, Dubourg L, Liutkus A, Hadj-Haïssa A, Rivet C, Boillot O, Lachaux A, Cochat P. Renal function in pediatric liver transplantation: a long-term follow-up study. Transplantation. 2008;86:1028-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Barshes NR, Lee TC, Udell IW, O'mahoney CA, Karpen SJ, Carter BA, Goss JA. The pediatric end-stage liver disease (PELD) model as a predictor of survival benefit and posttransplant survival in pediatric liver transplant recipients. Liver Transpl. 2006;12:475-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Knaup E, Nosaka N, Yorifuji T, Tsukahara K, Naito H, Tsukahara H, Nakao A; JaRPAC Study Group. Long-stay pediatric patients in Japanese intensive care units: their significant presence and a newly developed, simple predictive score. J Intensive Care. 2019;7:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Marcin JP, Slonim AD, Pollack MM, Ruttimann UE. Long-stay patients in the pediatric intensive care unit. Crit Care Med. 2001;29:652-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Niewińsk G, Raszeja-Wyszomirska J, Główczyńska R, Figiel W, Zając K, Kornasiewicz O, Zieniewicz K, Grąt M. Risk Factors of Prolonged ICU Stay in Liver Transplant Recipients in a Single-Center Experience. Transplant Proc. 2018;50:2014-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Otte JB. Pediatric liver transplantation: Personal perspectives on historical achievements and future challenges. Liver Transpl. 2016;22:1284-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Cottis R, Magee N, Higgins DJ. Haemodynamic monitoring with pulse-induced contour cardiac output (PiCCO) in critical care. Intensive Crit Care Nurs. 2003;19:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Nacoti M, Corbella D, Fazzi F, Rapido F, Bonanomi E. Coagulopathy and transfusion therapy in pediatric liver transplantation. World J Gastroenterol. 2016;22:2005-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |