-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Laura Gennaro, Immunologic Diagnosis of Tuberculosis, Clinical Infectious Diseases, Volume 30, Issue Supplement_3, June 2000, Pages S243–S246, https://doi.org/10.1086/313868
Close - Share Icon Share
Abstract
Evaluation of new vaccines against tuberculosis requires diagnostic tools for accurately identifying asymptomatic individuals infected with Mycobacterium tuberculosis and persons with active tuberculosis. This article discusses limitations of current methods for the immunologic diagnosis of latent infection and active disease and presents novel approaches to developing skin tests and serodiagnostic assays based on “cocktails” of multiple antigens of M. tuberculosis.
In order to evaluate new antituberculosis vaccines, diagnostic tools are needed for distinguishing noninfected persons, nonsymptomatic persons infected with Mycobacterium tuberculosis, and persons with active tuberculosis. Some of the limitations of current immunologic methods for the diagnosis of tuberculosis reflect the diverse recognition of M. tuberculosis antigens caused by differences in patients' immunogenetics and the stage-specificity of antigen recognition. Assays utilizing “cocktails” of antigens should effectively cover the diversity of immune responses and provide more accurate tools for immunologic diagnosis of tuberculosis. Recent progress in the development of cocktails of antigens for tuberculosis skin testing and serodiagnostic assays is discussed.
Identification of Infected Individuals
Accurate identification of individuals infected with M. tuberculosis is critical for the success of a vaccine trial for several reasons. First, selection of a vaccine trial site entails knowledge about the burden of tuberculosis, i.e., the proportion of infected individuals in the population of the region. Second, vaccine evaluation usually requires an accurate estimate of the number of infected individuals among those participating in the trial. This is particularly true for a preexposure vaccine, whose performance is evaluated on the basis of the number of individuals infected postvaccination. Third, evaluation of the impact of vaccination on the incidence of tuberculosis requires reassessment of the tuberculosis burden after vaccine implementation. Thus, the ability to diagnose infection with M. tuberculosis in vaccine recipients is critical to proper vaccine evaluation.
The requirements of a vaccine trial cannot be successfully met with use of the current tuberculin skin test, whose applications and limitations are reviewed by Shinnick in this issue (see pp. S276–8). In brief, the highly cross-reactive nature of the PPD of tuberculin confounds the interpretation of skin-test positivity for individuals who have a history of vaccination with Mycobacterium bovis BCG. This is also true for persons living in or originating from geographic areas with a high environmental load of nontuberculous mycobacteria [1, 2]. Thus, new skin-test reagents are needed that are specific for tuberculosis.
Development of tuberculosis-specific tuberculins entails a 2-step approach. First, M. tuberculosis-specific antigens that elicit delayed-type hypersensitivity (DTH) responses must be identified. Many such antigens have been recently described (a partial list is presented in table 1), and additional ones will certainly be discovered at a faster pace now that the genomic nucleotide sequence of M. tuberculosis has become available [12].
A second requirement is the formulation of cocktails of multiple purified antigens as skin-test reagents. A need for multiple antigens was suggested by the observation that a single potent species-specific antigen, such as MPT64 [13], fails to elicit DTH responses in most PPD-positive subjects tested [14]. Unlike single proteins, multiantigen formulations can presumably recruit many antigen-specific T cells to the site of antigen injection to generate a skin reaction of the appropriate size. Moreover, numerous antigens may be required to overcome problems related to genetic restriction of antigen recognition, a phenomenon that causes some individuals to react to certain antigens and not to others.
Guinea pig studies have provided several lines of evidence that cocktails of multiple purified antigens of M. tuberculosis should be ideal skin-test reagents. First, the skin-test activity of a cocktail was found to be greater than that of any single antigen, and it increased with the number of antigens in the cocktail. The increase occurred even when the same amount of total protein was used for the cocktail and for each single antigen [8]. These results establish that a multiantigen cocktail is significantly more active in tuberculosis skin-tests than any of the single antigens in the cocktail.
Second, the number of responders to a 2-antigen cocktail was greater than the number of responders to either antigen alone [3]. Thus, a multiantigen cocktail should cover the breadth of responses to M. tuberculosis antigens. Third, a cocktail of M. tuberculosis-complex-specific antigens was shown to elicit DTH responses in guinea pigs immunized with M. bovis BCG, but not in M. avium-immunized animals (figure 1 and [8]). Thus, a cocktail retains the specificity to tubercle bacilli of the antigens in the cocktail.
Skin-test reactivity of multiantigen cocktails in guinea pigs immunized with Mycobacterium bovis BCG (white bars) and with M. avium (shaded bars). Groups of 6 outbred female guinea pigs were sensitized by intradermal injection in the abdomen with 107 live M. bovis BCG Japanese or M. avium cells. Five weeks after sensitization, animals were intradermally injected with 10 TU of PPD and 8 µg of 4-antigen cocktails. Results are expressed as diameters (in mm) of skin reactions measured 24 h following antigen injection. M. tuberculosis complex-specific antigens were MPT63, MPT64, MTC28, and MPT70. Cross-reactive antigens were MPT51, Ag85B, MPT32, and KatG. B-PPD, M. bovis PPD; A-PPD, M. avium PPD. (Figure is modified from [8].)
The design of tuberculins of defined composition should also enable us to develop a skin test that can distinguish between infection with M. tuberculosis and vaccination with M. bovis BCG. Toward this end, it is necessary to identify DTH-eliciting antigens encoded by genome regions that are present in virulent M. tuberculosis but not in M. bovis BCG [15]. Likewise, if a subunit vaccine were to be developed against tuberculosis, diagnostic skin-test reagents should be based on antigens not included in the vaccine formulation.
Identification of Individuals with Active Disease
Vaccine evaluation requires tools for accurate diagnosis of active tuberculosis both during a vaccine trial (to identify individuals who develop active tuberculosis among those participating in the trial) and after vaccine implementation (to evaluate the impact of vaccination on tuberculosis incidence). Suspicion of active disease is commonly derived from clinical symptoms and chest radiographs, which have low diagnostic specificity, whereas confirmation of diagnosis is based on microbiological methods. The limitations of the microbiological methods used to diagnose tuberculosis are reviewed by Shinnick in this issue (see pp. S276–8).
In brief, microscopic examination of sputum smears for detection of acid-fast bacilli (AFB microscopy) has a low diagnostic sensitivity, particularly when the number of specimens exceeds the capacity of the laboratory. Culture methods are extremely slow, and their execution requires good laboratory facilities (for a recent review, see [16]). The limitations of microbiological methods for the diagnosis of tuberculosis may not apply to a vaccine trial, which is usually supported by state-of-the-art diagnostic facilities. Even in satisfactory laboratory settings, however, methods based on detection of tubercle bacilli or their products are inadequate for cases of paucibacillary pulmonary tuberculosis and extrapulmonary tuberculosis. Moreover, the ability to diagnose tuberculosis accurately needs to be maintained once vaccination is fully implemented, to enable evaluation of the long-term impact of vaccination on tuberculosis incidence. Thus, simple and accurate diagnostic methods are needed even in the context of vaccine evaluation.
Assays based on the detection of immunologic responses to tuberculosis are attractive alternatives to current methods for diagnosing active tuberculosis because they do not depend on detection of mycobacteria. Among the immunodiagnostic assays, methods based on detection of specific antibodies in serum or other biological fluids are promising for several reasons. One is the fact, first recognized in 1898 [17], that strong antibody responses are mounted during tuberculosis. This finding provides the basis for a serological approach to diagnosis of tuberculosis.
Second, serological methods can be simple, rapid, inexpensive, and relatively noninvasive. Third, serological methods, unlike tuberculin skin-testing, can potentially distinguish between active disease and nonsymptomatic infection, because specific antibody responses are rarely detected in nonsymptomatic individuals whose tuberculin skin-test is positive ([18] and our unpublished observations). Thus, serological testing has the potential of becoming a useful diagnostic tool for the rapid diagnosis of active tuberculosis.
The history of serological studies in the field of tuberculosis demonstrates that an inadequate knowledge of humoral immune responses during tuberculosis has generally led to unsatisfactory serodiagnostic methods. Researchers in tuberculosis immunology have paid little attention to humoral immunity because antibodies do not confer protective immunity to tuberculosis. Traditionally, most of our knowledge on antibody responses during tuberculosis has been derived from serodiagnostic studies. In the process of selecting purified antigens for serodiagnostic purposes (highly cross-reactive complex antigen mixtures yielded reactions of poor specificity [19–21]), it was repeatedly observed that single-antigen-based assays never achieved satisfactory serodiagnostic performance (reviewed in [21, 22]).
For example, assays based on the serologically immunodominant 38-kDa antigen [23] attained high sensitivity (∼80%) with sputum smear-positive pulmonary tuberculosis but performed poorly with sputum smear-negative cases (15% sensitivity; reviewed in [21]). Sera lacking anti-38-kDa antibodies were thought to be highly unlikely to react with any other antigen of M. tuberculosis [24]. From these observations, it was surmised that up to 30% of tuberculosis cases were seronegative [25].
Our current understanding of the antibody response during tuberculosis is derived from serological studies utilizing multiple purified protein antigens of M. tuberculosis. We have shown that the antibody response during tuberculosis is directed against many mycobacterial antigens and is almost universal (at least 90% of tuberculosis patients produce specific IgG serum antibodies). Moreover, antigen recognition during tuberculosis is highly heterogeneous, for no single antigen or common set of antigens is recognized by serum antibodies from all (or even most) patients [26].
These findings imply that the poor performance of single-antigen assays did not reflect a lack of antibody response in a large proportion of patients but rather a lack of appropriate reagents to measure response; multiple rather than single purified antigens must be used for diagnosis of tuberculosis by serological methods. The requirement of combinations of antigens to increase sensitivity of tuberculosis serodiagnostic methods has also been suggested by other investigators [27, 28]. An additional argument in favor of the development of multiantigen cocktails for serodiagnosis of tuberculosis lies in the intriguing possibility that recognition of certain antigens correlates with the stage of tuberculosis [29, 30]. Thus, development of serological methods for diagnosis of tuberculosis requires identification of antigens that cover the spectrum of responses in terms of stage specificity as well as individual-to-individual variability.
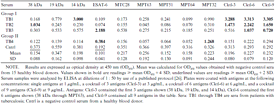
The development of multiantigen cocktails for serodiagnosis of tuberculosis requires that behavior in the solid phase be defined for antigens in combination. Our recent work has begun to elucidate how cocktails of multiple antigens perform in an ELISA. First, nonspecific background binding to a multiantigen cocktail does not exceed that measured with each antigen when tested singly (table 2; compare mean optical density (450 nm) values obtained with negative control sera tested with single antigens and multiantigen cocktails). Thus, cocktail-based assays should not sustain loss of diagnostic accuracy caused by elevated background readings.
Reactivity of selected sera to single antigens and multiantigen “cocktails.”
Second, high-level serum reactivities to a single antigen in the cocktail are sufficient to yield diagnostic positivity to cocktails of multiple antigens (as exemplified by sera in table 2, group I). Third, low-level reactivities in sera from tuberculosis patients and in control sera to one or more antigens are lost when using cocktails (as exemplified by sera in table 2, group II). This observation suggests that false-positive reactions caused by low-level reactivities in tuberculosis-negative sera should be lost when a multiantigen cocktail is used, with a gain of diagnostic specificity.
However, there may also be a loss of diagnostic sensitivity associated with low-level reactivities in sera from tuberculosis patients. The reduction in sensitivity may range between 10% and 25%, depending on the number of antigens in the cocktail and the total amount of protein used for coating (author's unpublished observations). To overcome limitations of ELISA in the use of multiantigen cocktails, we are currently evaluating alternative solid-phase supports, such as nitrocellulose membranes, which have a higher protein-binding capacity than the microtiter plate well.
Conclusions
Development of immunologic assays for the diagnosis of tuberculosis infection and disease requires that we understand the immunologic responses of interest and identify antigens specific for M. tuberculosis. Multiple antigens are needed because antigen recognition in tuberculosis is broad and highly variable from individual to individual and may be stage specific. On the basis of our recent understanding of the performance of multiantigen cocktails in skin-tests and serological assays, it is expected that multiantigen cocktails will yield a new generation of immunodiagnostic assays for tuberculosis that are useful in vaccine trials.
Acknowledgments
The author is grateful to the meeting organizers, Ann M. Ginsberg and Robert F. Breiman, for the opportunity to participate in the International Symposium on Tuberculosis Vaccine Development and Evaluation, held 26–28 August 1998 in San Francisco; Konstantin Lyashchenko and Roberto Colangeli for contributing to many of the ideas presented; Yvan P. Côté for sharing single-antigen and multiantigen ELISA data; Morten Harboe for his advice on table 1; and Karl Drlica for his comments on the manuscript.
References
Grant support: Research on immunologic diagnosis of tuberculosis in the author's laboratory is supported by National Institutes of Health grant AI-36989.




![Skin-test reactivity of multiantigen cocktails in guinea pigs immunized with Mycobacterium bovis BCG (white bars) and with M. avium (shaded bars). Groups of 6 outbred female guinea pigs were sensitized by intradermal injection in the abdomen with 107 live M. bovis BCG Japanese or M. avium cells. Five weeks after sensitization, animals were intradermally injected with 10 TU of PPD and 8 µg of 4-antigen cocktails. Results are expressed as diameters (in mm) of skin reactions measured 24 h following antigen injection. M. tuberculosis complex-specific antigens were MPT63, MPT64, MTC28, and MPT70. Cross-reactive antigens were MPT51, Ag85B, MPT32, and KatG. B-PPD, M. bovis PPD; A-PPD, M. avium PPD. (Figure is modified from [8].)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/30/Supplement_3/10.1086_313868/1/m_30-Supplement_3-S243-fig001.gif?Expires=1716392026&Signature=D3LCS9UiPpUtuYU69fwK4FYmi4IJrk3eUVb24epIOcBx3cgPGuHZHAJ7G1edGn4eNhw4JZC1l0A8fnOrSIj3~euUtHcQ2L7bfFqkQYed4ppnr6F2~agEiY6NHxBxF4jovSxeUdPg3ETGYQ16~i7dxIEGSAW6lWx078tq7kyn8ZBiAuIUpqnpiME3MBl0btt3mj9SvI15n~dJaa57Olv5UVZPJx8qCV6rcKm8iYCb8GdjuXI7tM-yOH6T96eHNEMPuzNdihwQtdDi97kPvrr3iS9YINeLfoM3oJ6Gzbq~kQLFMDLDIDYPiu8azX~24NFvnObrWSAteTo6MWVBV~ZfCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments