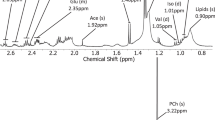
Abstract
Imaging is a key component in the management of brain tumours, with MRI being the preferred modality for most clinical scenarios. However, although conventional MRI provides mainly structural information, such as tumour size and location, it leaves many important clinical questions, such as tumour type, aggressiveness and prognosis, unanswered. An increasing number of studies have shown that additional information can be obtained using functional imaging methods (which probe tissue properties), and that these techniques can give key information of clinical importance. These techniques include diffusion imaging, which can assess tissue structure, and perfusion imaging and magnetic resonance spectroscopy, which measures tissue metabolite profiles. Tumour metabolism can also be investigated using PET, with 18F-deoxyglucose being the most readily available tracer. This Review discusses these methods and the studies that have investigated their clinical use. A strong emphasis is placed on the measurement of quantitative parameters, which is a move away from the qualitative nature of conventional radiological reporting and presents major challenges, particularly for multicentre studies.
Key Points
-
Conventional imaging gives information largely on tumour structure and location and is increasingly being supplemented by methods that probe tissue properties, commonly referred to by the collective term 'functional imaging'
-
A range of functional imaging techniques for brain tumours that provide information on cellularity, tissue ultrastructure, metabolism and vascularity are available and best acquired as part of a multimodal protocol
-
Increasing evidence shows that these techniques can aid diagnosis, provide noninvasive prognostic biomarkers, help treatment planning and monitor the treatment of brain tumours
-
Challenges remain in determining the optimum manner for incorporating these techniques into routine clinical practice, and robust data for their role should be obtained from multicentre clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yousem, D. M., Zimmerman, R. D. & Grossman, R. I. Neuroradiology: The Requisites, 3rd edn (Mosby, Philadelphia, 2010).
Julia-Sape, M. et al. Prospective diagnostic performance evaluation of single voxel (1) MRS for typing and grading of brain tumours. NMR Biomed. 25, 661–673 (2012).
Price, S. J. et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. Am. J. Neuroradiol. 27, 1969–1974 (2006).
Brandsma, D., Stalpers, L., Taal, W., Sminia, P. & van den Bent, M. J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 9, 453–461 (2008).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
Stockhalm, A. L. et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J. Neurooncol. 109, 149–158 (2012).
Merboldt, K. D., Hänicke, W., Bruhn, H., Gyngell, M. L. & Frahm, J. Diffusion imaging of the human brain in vivo using high-speed STEAM MRI. Magn. Reson. Med. 23, 179–192 (1992).
Le Bihan, D. et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161, 401–407 (1986).
McRobbie, D. W., Moore, E. A., Graves, M. J. & Prince, M. R. MRI From Picture To Proton, 2nd edn (Cambridge University Press, Cambridge, 2007).
Gillard, J. H., Waldman, A. D. & Barker, P. B. (Eds) Clinical MR Neuroimaging Physiological and Functional Techniques. (Cambridge University Press, Cambridge, 2007).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).
Rosen, B. R., Belliveau, J. W. & Chien, D. Perfusion imaging by nuclear magnetic resonance. Magn. Reson. Q. 5, 263–281 (1989).
Hylton, N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J. Clin. Oncol. 24, 3293–3298 (2006).
Carr, D. H. et al. Intravenous chelated gadolinium as a contrast agent in NMR imaging of cerebral tumours. Lancet 1, 484–486 (1984).
Parker, G. J. et al. Probing tumor microvascularity by measurement, analysis and display of contrast agent uptake kinetics. J. Magn. Reson. Imaging 7, 564–574 (1997).
Tofts, P. S. & Kermode, A. G. Measurement of the blood–brain barrier permeability and leakage space using dynamic MR Imaging 1. Fundamental concepts. Magn. Reson. Med. 17, 357–367 (1991).
Kiselev, V. G. On the theoretical basis of perfusion measurements by dynamic susceptibility contrast MRI. Magn. Reson. Med. 46, 1113–1122 (2001).
Villringer, A. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn. Reson. Med. 6, 164–174 (1998).
Leach, M. O. et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br. J. Cancer. 92, 1599–1610 (2005).
Leach, M. O. et al. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur. Radiol. 22, 1451–1464 (2012).
O'Connor, J. P. et al., Dynamic contrast-enhanced imaging techniques: CT and MRI. Br. J. Radiol. 84 (Suppl. 2), S112–S120 (2011).
Peet, A. C. et al. The value of magnetic resonance spectroscopy in tumour imaging. Arch. Dis. Chil. 93, 725–727 (2008).
Kries, R. Issues of spectral quality in clinical 1H magnetic resonance spectroscopy and a gallery of artefacts. NMR Biomed. 17, 361–381 (2004).
Preul, M. C. et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat. Med. 2, 323–325 (1996).
Garcia-Gómez, J. M. et al. Multiproject-multicentre evaluation of automatic brain tumour classification by magnetic resonance spectroscopy. MAGMA 22, 5–18 (2009).
Scheidegger, O. et al. Optimised quantitative magnetic resonance spectroscopy for clinical routine. Magn. Reson Med. http://dx.doi.org/10.1002/mrm.24455
Scott, A. M. Current status of positron emission tomography in oncology. Intern. Med. J. 31, 27–36 (2001).
Kovanlikaya, A. et al. Untreated pediatric primitive neuroectodermal tumour in vivo: quantitation of taurine with MR spectroscopy. Radiology 236, 1020–1025 (2005).
Astrakas, L. G. et al. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin. Cancer Res. 10, 8220–8228 (2004).
Davies, N. P. et al. Non-invasive detection of glycine as a biomarker of malignancy in childhood brain tumours using in-vivo1H MRS at 1.5 Tesla and ex-vivo high-resolution magic-angle spinning NMR. NMR Biomed. 23, 80–87 (2010).
Panigraphy, A. et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: Preoperative diagnosis and characterization. AJNR Am. J. Neuroradiol. 27, 560–572 (2006).
Tate, A. R. et al. Development of a decision support system for diagnosis and grading of brain tumours using in-vivo magnetic resonance single voxel spectra. NMR Biomed. 19, 411–434 (2006).
Davies, N. P. et al. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS using discriminant analysis of metabolite profiles. NMR Biomed. 21, 908–918 (2008).
Vicente, J. et al. Accurate classification of childhood brain tumours by in vivo1H MRS—a multi-centre study, Eur. J. Cancer doi:10.1016/j.ejca.2012.09.003.
Perez-Ruiz, A. et al. The INTERPRET Decision Support System version 3.0 for evaluation of Magnetic Resonance Spectroscopy data from human brain tumours and other abnormal brain masses. BMC Bioinformatics 22, 581 (2010).
Higano, S. et al. Malignant astrocytic tumours: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241, 839–846 (2006).
Rumboldt, Z., Camacho, D. L., Lake, D., Welsh, C. T. & Castillo, M. Apparent diffusion coefficients for differentiation of cerebellar tumours in children. AJNR Am. J. Neuroradiol. 27, 1362–1369 (2006).
Kang, Y. et al. Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion weighted MR imaging correlation with tumour grade. Radiology 261, 882–890 (2011).
Server, A. et al. Quantitative apparent diffusion coefficient in the characterisation of brain tumours and associated peritumoural edema. Acta Radiol. 50, 682–689 (2009).
Jaremko, J. L., Jans, L. B., Coleman, L. T. & Ditchfield, M. R. Value and limitations of diffusion weighted imaging in grading and diagnosis of pediatric posterior fossa tumours. AJNR Am. J. Neuroradiol. 31, 1613–1616 (2010).
Tozer, D. J. et al. Apparent diffusion coefficient histograms may predict low grade glioma subtype. NMR Biomed. 20, 49–57 (2007).
Bull, J. G., Saunders, D. E. & Clark, C. A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 22, 447–457 (2012).
Jolapara, M. et al. Can diffusion tensor metrics help in preoperatively grading of diffusely infiltrating astrocytomas? A retrospective study of 36 cases. Neuroradiology 53, 63–68 (2011).
White, M. L., Zhang, Y., Yu, F. & Jaffar Kazmi, S. A. Diffusion tensor MR imaging of cerebral gliomas: evaluating fractional anisotropy characteristics. AJNR Am. J. Neuroradiol. 32, 374–381 (2011).
Brynes, T. J., Barrick, T. R., Bell, B. A. & Clark, C. A. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed. 24, 54–60 (2011).
Dunn, G. P. et al. Emerging Insights into the molecular and cellular basis of glioblastoma. Genes Dev. 26, 756–784 (2012).
Sugahara, T. et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am. J. Roentgenol. 171, 1479–1486 (1998).
Arvinda, H. R. et al. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J. Neurooncol. 94, 87–96 (2009).
Law, M. et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 24, 1989–1998 (2003).
Patankar, T. F. et al. Is volume transfer coefficient (Ktrans) related to histologic grade in human gliomas? AJNR Am. J. Neuroradiol. 26, 2455–2465 (2005).
Pauleit, D. et al. Comparison of (18)F-FET and (18)F-FDG PET in brain tumours. Nucl. Med. Biol. 36, 779–787 (2009).
Peet, A. C. et al. Magnetic resonance spectroscopy suggests key differences in the metastatic behaviour of medulloblastoma. Eur. J. Cancer. 43, 1037–1044 (2007).
Jenkinson, M. et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 48, 703–713 (2006).
Choi, C. et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 18, 624–629 (2012).
Server, A. et al. Proton magnetic resonance spectroscopy in the distinction of high grade cerebral gliomas from single metastatic brain tumours. Acta Radiol. 51, 316–325 (2010).
Wright, A. J. et al. Pattern recognition of MRSI data shows regions of glioma growth that agree with DTI markers of brain tumour invasion. Magn. Reson. Med. 62, 1646–1651 (2009).
Fellows, G. A. et al. Combined use of neuroradiology and 1H-MR spectroscopy may provide an intervention limiting diagnosis of glioblastoma multiforme. J. Magn. Reson. Imaging. 32, 1038–1044 (2010).
Weber, M. A. et al. Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Invest. Radiol. 45, 755–768 (2010).
Widhalm, G. et al. Value of 1H-magnetic resonance spectroscopy chemical shift imaging for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast-enhancement. J. Neurol. Psychiatry. 82, 512–520 (2010).
Law, M. et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247, 490–498 (2008).
Danchaivijitr, N. et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology 247, 170–178 (2008).
Majos, C. et al. Proton MR spectroscopy provides relevant prognostic information in high grade gliomas. AJNR Am. J. Neuroradiol. 32, 74–80 (2011).
Marcus, K. J. et al. Predicting survival of children with CNS tumours using proton magnetic resonance spectroscopic imaging biomarkers. Int. J. Oncol. 30, 651–657 (2007).
Harris, L. M. et al. Magnetic resonance spectro-scopy in the assessment of pilocytic astrocytomas. Eur. J. Cancer. 44, 2640–2647 (2008).
Hattingen, E. et al. 1H MRSI and progression-free survival in patients with WHO grades II and III gliomas. Neurol. Res. 32, 593–602 (2010).
Hipp, S. J. et al. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol. 13, 904–909 (2011).
Blüml, S. et al. Elevated citrate in pediatric astrocytomas with malignant progression. Neuro. Oncol. 13, 1107–1117 (2011).
Pope, W. B. et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252, 182–189 (2009).
Bagadia, A., Purandare, H., Misra, B. K. & Gupta, S. Application of magnetic resonance tractography in the perioperative planning of patients with eloquent region intra-axial brain lesions. J. Clin. Neurosci. 18, 633–639 (2011).
Romano, A. et al. Pre-surgical planning and MR-tractography utility in brain tumour resection. Euro. Radiol. 19, 2798–2808 (2009).
Gulati, S. et al. Surgical resection of high-grade gliomas by blood oxygen level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperaive navigated 3D ultrasound. Minim. Invasive Neurosurg. 52, 17–24 (2009).
D'Andrea, G. et al. Intraoperative DTI and brain mapping for surgery of neoplasm of the motor cortex and the corticospinal tract: our protocol and series in BrainSUITE. Neurosurg. Rev. 35, 401–412 (2012).
Pilli, J. J. The evolution of clinical functional imaging during the past 2 decades and its current impact on neurosurgical planning. AJNR Am. J. Neuroradiol. 31, 219–225 (2010).
Bartos, R. et al. Validity of primary motor area localization with fMRI versus electrical cortical stimulation: a comparative study. Acta Neurochir. (Wien) 151, 1071–1080 (2009).
Amiez, C. et al. Preoperative functional magnetic resonance imaging assessment of higher-order cognitive function in patients undergoing surgery for brain tumors. J. Neurosurg. 108, 258–268 (2008).
Ballangrud, A. M. et al. Magnetic resonance spectroscopy imaging in radiotherapy planning for recurrent glioma. Med. Phys. 38, 2724–2730 (2011).
Pirzkall, A. et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro. Oncol. 11, 842–852 (2009).
Rosenschöld, P. M. et al. Photon and proton therapy planning comparison for malignant glioma based on, CT, FDG-PET, DTI-MRI and fibre tracking. Acta Oncol. 50, 777–783 (2011).
Einstein, D. B. et al. Phase II Trial of radiosurgery to magnetic resonance spectroscopy-defined high risk tumour volumes in patients with glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 84, 668–674 (2012).
Kovács, Á. et al., Integrating functional MRI information into radiotherapy planning of CNS tumors-early experiences. Pathol. Oncol. Res. 17, 207–217 (2011).
van den Bent, M. J. et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 12, 583–593 (2011).
Cao, Y. et al. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int. J. Radiat. Oncol. Biol. Phys. 64, 876–885 (2006).
Galbán, C. J. et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat. Med. 15, 572–576 (2009).
Alexander, A. et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiotherapy for patients with malignant glioma. Clin. Invest. Med. 29, 301–311 (2006).
Quon, H. et al. Changes in serial magnetic resonance spectroscopy predict outcome in high-grade glioma during and after postoperative radiotherapy. Anticancer Res. 31, 3559–3565 (2011).
Guillevin, R. et al. Predicting the outcome of grade II glioma treated with temozolomide using proton magnetic resonance spectroscopy. Br. J. Cancer. 104, 1854–1861 (2011).
Nowosielski, M. et al. ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53, 291–302 (2011).
Hamstra, D. A. et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J. Clin. Oncol. 26, 3387–3394 (2008).
Hargrave, D., Chuang, N. & Bouffet, E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J. Neurooncol. 86, 313–319 (2008).
Nicolin, G. et al. Natural history and outcome of optic pathway gliomas in children. Pediatr. Blood Cancer 53, 1231–1237 (2009).
Bradley, D. P. et al. Examining the acute effects of cediranib (RECENTIN, AZD2171) treatment in tumour models: a dynamic contrast-enhanced MRI study using gadopentate. Magn. Reson. Imaging. 27, 377–384 (2009).
Sorensen, A. G. et al. A 'vascular normalization index' as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 69, 5296–5300 (2009).
Kim, H. et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 71, 3745–3752 (2011).
Hu, L. S. et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from post treatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am. J. Neuroradiol. 30, 552–558 (2009).
Barajas, R. F. Jr et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiotherapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253, 486–496 (2009).
Zeng, Q. S. et al. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J. Neurooncol. 84, 63–69 (2007).
Tsien, C. et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J. Clin. Oncol. 28, 2293–2299 (2010).
Prat, R. et al. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J. Clin. Neurosci. 17, 50–53 (2010).
Imani, F. et al. Comparison of proton magnetic resonance spectroscopy with fluorine-18 2-fluoro-deoxyglucose positron emission tomography for assessment of brain tumour progression. J. Neuroimaging 22, 184–190 (2012).
Gulyás, B. & Halldin, C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q. J. Nucl. Med. Imaging. 56, 173–190 (2012).
Grosu, A. L. et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 63, 64–74 (2005).
Galldiks, N. et al. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging. 33, 516–524 (2006).
Krishnan, A. S. et al. Detection of cell death in tumors by using MR imaging and a gadolinium-based targeted contrast agent. Radiology 246, 854–862 (2008).
Strijkers, G. J. et al. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 13, 161–173 (2010).
Neuner, I. et al. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur. Radiol. http://dx.doi.org/10.1007/s00330-012-2543-x
Day, S. E. et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat. Med. 13, 1382–1387 (2007).
Takasawa, M., Moustafa, R. R. & Baron, J. C. Applications of nitroimidazole in vivo hypoxia imaging in ischemic stroke. Stroke 39, 1629–1637 (2008).
Wang, W. et al. Pharmacokinetic analysis of Hypoxia 18F-fluoromisonidazole dynamic PET in head and neck cancer. J. Nucl. Med. 51, 37–45 (2010).
Zhou, J. et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 17, 130–134 (2011).
Functional Imaging Group [online].
Panigrahy, A., Nelson, M. D. Jr & Bluml, S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr. Radiol. 40, 3–30 (2010).
Rosso, L. et al. A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res. 69, 120–127 (2009).
Howe, F. A. & Opstad, K. S. 1H MR spectroscopy of brain tumours and masses. NMR Biomed. 16, 123–312 (2003).
Wilson, M. P., Reynolds, G., Kauppinen, R. A., Arvanitis, T. A. & Peet, A. C. A constrained least-squares approach to the automated quantitation of in-vivo 1H MRS data. Magn. Reson. Med. 65, 1–12 (2011).
Acknowledgements
A. C. Peet, T. N. Arvanitis and M. O. Leach would like to acknowledge funding from the Cancer Research UK and Engineering and Physical Sciences Research Council Cancer Imaging Programme at the Children's Cancer and Leukaemia Group in association with the Medical Research Council and Department of Health (England) (C7809/A10342). A. D. Waldman would like to acknowledge the support of Imperial College Comprehensive Biomedical Research Centre. We would like to thank the members of the Brain Tumour Research Group at the University of Birmingham who helped to prepare figures for the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided a substantial contribution to discussions of the content, contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Peet, A., Arvanitis, T., Leach, M. et al. Functional imaging in adult and paediatric brain tumours. Nat Rev Clin Oncol 9, 700–711 (2012). https://doi.org/10.1038/nrclinonc.2012.187
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.187
This article is cited by
-
Posterior fossa tumors in children: current insights
European Journal of Pediatrics (2023)
-
Joint EANM/SIOPE/RAPNO practice guidelines/SNMMI procedure standards for imaging of paediatric gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
The role of Magnetic Resonance Images (MRIs) in coping for patients with brain tumours and their parents: a qualitative study
BMC Cancer (2021)
-
Classification of paediatric brain tumours by diffusion weighted imaging and machine learning
Scientific Reports (2021)
-
Role of diffusion-weighted imaging in differentiation between posterior fossa brain tumors
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2020)