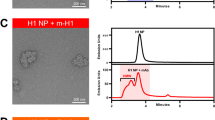
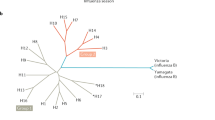
Abstract
Influenza viruses pose a significant threat to the public and are a burden on global health systems1,2. Each year, influenza vaccines must be rapidly produced to match circulating viruses, a process constrained by dated technology and vulnerable to unexpected strains emerging from humans and animal reservoirs. Here we use knowledge of protein structure to design self-assembling nanoparticles that elicit broader and more potent immunity than traditional influenza vaccines. The viral haemagglutinin was genetically fused to ferritin, a protein that naturally forms nanoparticles composed of 24 identical polypeptides3. Haemagglutinin was inserted at the interface of adjacent subunits so that it spontaneously assembled and generated eight trimeric viral spikes on its surface. Immunization with this influenza nanoparticle vaccine elicited haemagglutination inhibition antibody titres more than tenfold higher than those from the licensed inactivated vaccine. Furthermore, it elicited neutralizing antibodies to two highly conserved vulnerable haemagglutinin structures that are targets of universal vaccines: the stem4,5 and the receptor binding site on the head6,7. Antibodies elicited by a 1999 haemagglutinin–nanoparticle vaccine neutralized H1N1 viruses from 1934 to 2007 and protected ferrets from an unmatched 2007 H1N1 virus challenge. This structure-based, self-assembling synthetic nanoparticle vaccine improves the potency and breadth of influenza virus immunity, and it provides a foundation for building broader vaccine protection against emerging influenza viruses and other pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Salomon, R. & Webster, R. G. The influenza virus enigma. Cell 136, 402–410 (2009)
Lambert, L. C. & Fauci, A. S. Influenza vaccines for the future. N. Engl. J. Med. 363, 2036–2044 (2010)
Yamashita, I., Iwahori, K. & Kumagai, S. Ferritin in the field of nanodevices. Biochim. Biophys. Acta 1800, 846–857 (2010)
Ekiert, D. C. et al. Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009)
Sui, J. et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nature Struct. Mol. Biol. 16, 265–273 (2009)
Whittle, J. R. et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl Acad. Sci. USA 108, 14216–14221 (2011)
Ekiert, D. C. et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 489, 526–532 (2012)
Wei, C. J. et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064 (2010)
Ledgerwood, J. E. et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect. Dis. 11, 916–924 (2011)
Lee, L. A. & Wang, Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine 2, 137–149 (2006)
Li, C. Q., Soistman, E. & Carter, D. C. Ferritin nanoparticle technology.Anew platform for antigen presentation and vaccine development. Ind. Biotechnol. 2, 143–147 (2006)
Meldrum, F. C., Heywood, B. R. & Mann, S. Magnetoferritin: in vitro synthesis of a novel magnetic protein. Science 257, 522–523 (1992)
Jääskeläinen, A. et al. Production of apoferritin-based bioinorganic hybrid nanoparticles by bacterial fermentation followed by self-assembly. Small 3, 1362–1367 (2007)
Cho, K. J. et al. The crystal structure of ferritin from Helicobacter pylori reveals unusual conformational changes for iron uptake. J. Mol. Biol. 390, 83–98 (2009)
O'Hagan, D. T., Ott, G. S., Nest, G. V., Rappuoli, R. & Giudice, G. D. The history of MF59 adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines 12, 13–30 (2013)
Mbow, M. L., De Gregorio, E., Valiante, N. M. & Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 22, 411–416 (2010)
Nabel, G. J. & Fauci, A. S. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nature Med. 16, 1389–1391 (2010)
Okuno, Y., Isegawa, Y., Sasao, F. & Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67, 2552–2558 (1993)
Corti, D. et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–1673 (2010)
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011)
Ekiert, D. C. et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333, 843–850 (2011)
Krause, J. C. et al. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of influenza H1N1 virus hemagglutinin. J. Virol. 85, 10905–10908 (2011)
Lingwood, D. et al. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature 489, 566–570 (2012)
Bachmann, M. F. & Zinkernagel, R. M. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15, 235–270 (1997)
Xiong, A. S. et al. PCR-based accurate synthesis of long DNA sequences. Nature Protocols 1, 791–797 (2006)
Kong, W. P. et al. Protective immunity to lethal challenge of the 1918 pandemic influenza virus by vaccination. Proc. Natl Acad. Sci. USA 103, 15987–15991 (2006)
Wei, C. J. et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82, 6200–6208 (2008)
Wei, C. J. et al. Cross-neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci. Transl. Med. 2, 24ra21 (2010)
Yang, Z. Y. et al. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317, 825–828 (2007)
Wu, X. et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861 (2010)
Zhou, T. et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817 (2010)
Kong, W. P. et al. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J. Virol. 77, 12764–12772 (2003)
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Acknowledgements
We thank H. Andersen, A. Taylor, A. Zajac and C. Chiedi for help with the animal studies; U. Baxa, K. Nagashima and A. Harned for electron microscopy studies; X. Chen for technical support; A. Panet, B. Graham, R. Schwartz and members of the Nabel lab for discussions; S. Sun and M. Rossmann for technical and conceptual advice; A. Tislerics, B. Hartman and J. Farrar for manuscript preparation. The MF59 adjuvant was kindly provided by Novartis. This work was supported by the Intramural Research Program of the Vaccine Research Center, NIAID, National Institutes of Health.
Author information
Authors and Affiliations
Contributions
M.K., J.C.B. and G.J.N. developed the concept of HA–ferritin nanoparticles; M.K., C.-J.W. and G.J.N. designed the research studies; M.K., C.-J.W., H.M.Y., P.M.M., J.C.B., J.R.R.W., W.-P.K., L.W. and G.J.N. performed the research and analysed data; M.K., C.-J.W., H.M.Y., P.M.M., J.C.B., J.R.R.W. and G.J.N. discussed the results and implications; S.S.R. assisted in animal studies and sample collection; M.K., C.-J.W., J.C.B. and G.J.N. wrote the paper and all authors participated in manuscript revisions.
Corresponding author
Ethics declarations
Competing interests
G.J.N. is currently an employee of Sanofi, which produces commercial influenza vaccines. P.M.M. is currently an employee of MedImmune which also makes commercial influenza vaccines.
Additional information
The authors declare that an intellectual property application has been filed by NIH based on data presented in this paper.
Supplementary information
Supplementary Figures
This fie contains Supplementary Figures 1-7, which show a phylogenetic tree analysis and protein surface conservation between H. pylori and ferritins from other species; biochemical characterization of HA-nanoparticles; antigenic characterization of HA-nanoparticles; the effect of preexisting anti-ferritin immunity on subsequent immunization using HA-nanoparticles; iron incorporation by H. pylori ferritin and HA-nanoparticles; development of a trivalent vaccine comprising three independent HA-nanoparticles (H1, H3 and influenza B); and biochemical characterization of ΔRBS HA proteins. (PDF 775 kb)
Rights and permissions
About this article
Cite this article
Kanekiyo, M., Wei, CJ., Yassine, H. et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013). https://doi.org/10.1038/nature12202
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12202
This article is cited by
-
Development and application of an indirect ELISA for detection of antibodies against emerging atypical porcine pestivirus
Virology Journal (2024)
-
Enhancing antibody responses by multivalent antigen display on thymus-independent DNA origami scaffolds
Nature Communications (2024)
-
Diverse array of neutralizing antibodies elicited upon Spike Ferritin Nanoparticle vaccination in rhesus macaques
Nature Communications (2024)
-
A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response
Journal of Nanobiotechnology (2023)
-
Enhanced mucosal immune responses and reduced viral load in the respiratory tract of ferrets to intranasal lipid nanoparticle-based SARS-CoV-2 proteins and mRNA vaccines
Journal of Nanobiotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.