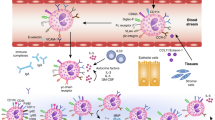
Abstract
Although eosinophils have been implicated in the pathogenesis of gastrointestinal disorders, their function has not been established. Using a murine model of oral antigen–induced eosinophil-associated gastrointestinal disease, we report the pathological consequences of eosinophilic inflammation and the involvement of eotaxin and eosinophils. Exposure of mice to enteric-coated antigen promotes an extensive T helper 2–associated eosinophilic inflammatory response involving the esophagus, stomach, small intestine and Peyer's patches as well as the development of gastric dysmotility, gastromegaly and cachexia. Electron microscopy shows eosinophils in proximity to damaged axons, which indicated that eosinophils were mediating a pathologic response. In addition, mice deficient in eotaxin have impaired eosinophil recruitment and are protected from gastromegaly and cachexia. These results establish a critical pathological function for eotaxin and eosinophils in gastrointestinal allergic hypersensitivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sampson, H. A. Food allergy. Part 1: immunopathogenesis and clinical disorders. J. Allergy Clin. Immunol. 103, 717–728 (1999).
Furuta, G. T., Ackerman, S. J. & Wershil, B. K. The role of the eosinophil in gastrointestinal diseases. Curr. Opin. Gastroenterol. 11, 541–547 (1995).
Sampson, H. A. Food allergy. J. Am. Med. Assoc. 278, 1888–1894 (1997).
Kelly, K. J. Eosinophilic gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 30 Suppl 28–35 (2000).
Gleich, G. J., Frigas, E., Loegering, D. A., Wassom, D. L. & Steinmuller, D. Cytoxic properties of the eosinophil major basic protein. J. Immunol. 123, 2925–2927 (1979).
Klein, N. C., Hargrove, R. L., Sleisenger, M. H. & Jeffries, G. H. Eosinophilic gastroenteritis. Medicine (Baltimore) 49, 299–319 (1970).
Cello, J. P. Eosinophilic gastroenteritis: a complex disease entity. Am. J. Med. 67, 1097–1114 (1979).
Torpier, G. et al. Eosinophilic gastroenteritis: ultrastructural evidence for a selective release of eosinophil major basic protein. Clin. Exp. Immunol. 74, 404–408 (1988).
Vandezande, L. M. et al. Interleukin-5 immunoreactivity and mRNA expression in gut mucosa from patients with food allergy. Clin. Exp. Allergy 29, 695–702 (1999).
Keshavarzian, A. et al. Activated eosinophils in familial eosinophilic gastroenteritis. Gastroenterology 88, 1041–1049 (1985).
Talley, N. J., Shorter, R. G., Phillips, S. F. & Zinsmeister, A. R. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 31, 54–58 (1990).
Desreumaux, P. et al. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis. Gastroenterology 110, 768–774 (1996).
Lowichik, A. & Weinberg, A. G. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod. Pathol. 9, 110–114 (1996).
Weller, P. F. et al. Role of the eosinophil in allergic reactions. Eur. Respir. J. 22, 109s–115s (1996).
Gleich, G. J. & Kita, H. Bronchial asthma: lessons from murine models. Proc. Natl Acad. Sci. USA 94, 2101–2102 (1997).
Schleimer, R. P. & Bochner, B. S. The role of adhesion molecules in allergic inflammation and their suitability as targets of antiallergic therapy. Clin. Exp. Allergy 28 Suppl 3, 15–23 (1998).
Cieslewicz, G. et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Invest. 104, 301–308 (1999).
Rothenberg, M. E. Eosinophilia. N. Engl. J. Med. 338, 1592–1600 (1998).
Gutierrez-Ramos, J. C., Lloyd, C. & Gonzalo, J. A. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic reactions. Immunol. Today 20, 500–504 (1999).
Garcia-Zepeda, E. A. et al. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nature Med. 2, 449–456 (1996).
Hogan, S. P., Mishra, A., Brandt, E. B., Foster, P. S. & Rothenberg, M. E. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc. Natl Acad. Sci. USA 97, 6681–6686 (2000).
Rothenberg, M. E. Eotaxin. An essential mediator of eosinophil trafficking into mucosal tissues. Am. J. Respir. Cell Mol. Biol. 21, 291–295 (1999).
Mishra, A., Hogan, S. P., Brandt, E. B. & Rothenberg, M. E. Peyer's patch eosinophils: identification, characterization, and regulation by mucosal allergen exposure, interleukin-5, and eotaxin. Blood 96, 1538–1544 (2000).
Mishra, A., Hogan, S. P., Lee, J. J., Foster, P. S. & Rothenberg, M. E. Fundamental signals regulate eosinophil homing to the gastrointestinal tract. J. Clin. Invest. 103, 1719–1727 (1999).
Litwin, A., Flanagan, M. & Michael, J. G. Microencapsulation. The future of oral immunotherapy? Biodrugs 9, 261–270 (1998).
Dvorak, A. M. et al. Ultrastructural identification of exocytosis of granules from human gut eosinophils in vivo. Int. Arch. Allergy Immunol. 102, 33–45 (1993).
Lloyd, C. M. et al. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 191, 265–274 (2000).
Grimaldi, J. C. et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3). J. Leukoc. Biol. 65, 846–853 (1999).
Collins, P. D., Marleau, S., Griffiths-Johnson, D. A., Jose, P. J. & Williams, T. J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182, 1169–1174 (1995).
Mestecky, J. et al. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J. Clin. Invest. 61, 731–737 (1978).
Michael, J. G. The role of digestive enzymes in orally induced immune tolerance. Immunol. Invest. 18, 1049–1054 (1989).
Jaffe, J. S. et al. Evidence for an abnormal profile of interleukin-4 (IL-4), IL-5, and gamma-interferon (gamma-IFN) in peripheral blood T cells from patients with allergic eosinophilic gastroenteritis. J. Clin. Immunol. 14, 299–309 (1994).
Gleich, G. J., Adolphson, C. R. & Leiferman, K. M. The biology of the eosinophilic leukocyte. Annu. Rev. Med. 44, 85–101 (1993).
Abu-Ghazaleh, R. I., Fujisawa, T., Mestecky, J., Kyle, R. A. & Gleich, G. J. IgA-induced eosinophil degranulation. J. Immunol. 142, 2393–2400 (1989).
Evans, C. M., Fryer, A. D., Jacoby, D. B., Gleich, G. J. & Costello, R. W. Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J. Clin. Invest. 100, 2254–2262 (1997).
Adamko, D. J., Yost, B. L., Gleich, G. J., Fryer, A. D. & Jacoby, D. B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 190, 1465–1478 (1999).
Lin, S. et al. Expression of muscarinic receptor subtypes in rat gastric smooth muscle: effect of M3 selective antagonist on gastric motility and emptying. Dig. Dis. Sci. 42, 907–914 (1997).
Talley, N. J., Kephart, G. M., McGovern, T. W., Carpenter, H. A. & Gleich, G. J. Deposition of eosinophil granule major basic protein in eosinophilic gastroenteritis and celiac disease. Gastroenterology 103, 137–145 (1992).
Stelts, D. et al. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 18, 463–470 (1998).
Erjefalt, J. S. & Persson, C. G. New aspects of degranulation and fates of airway mucosal eosinophils. Am. J. Respir. Crit. Care Med. 161, 2074–2085 (2000).
Mishra, A., Hogan, S. P., Brandt, E. B. & Rothenberg, M. E. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J. Clin. Invest. 107, 83–90 (2001).
Lee, R. G. Marked eosinophilia in esophageal mucosal biopsies. Am. J. Surg. Pathol. 9, 475–479 (1985).
Dobbins, J. W., Sheahan, D. G. & Behar, J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 72, 1312 (1977).
Kelly, K. J. et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 109, 1503–1512 (1995).
Woerly, G. et al. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma). Inhibition by immunoglobulin A complexes. J. Exp. Med. 190, 487–496 (1999).
Shi, H. Z., Humbles, A., Gerard, C., Jin, Z. & Weller, P. F. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J. Clin. Invest. 105, 945–953 (2000).
Rothenberg, M. E., MacLean, J. A., Pearlman, E., Luster, A. D. & Leder, P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 185, 785–790 (1997).
Acknowledgements
We thank A. Dvorak for review of the electron microscopy; M. Cohen, S. Wert, D. Witte, F. Finkelman and G. Khurana Hershey for discussions and review of the manuscript; A. Emley for graphic assistance; and I. Hofman for technical assistance with electron microscopy. Supported in part by the National Health Medical Research Council (Australia) C. J. Martin Postdoctoral Fellowship (to S. P. H.), National Institutes of Health grants R01 AI42242-02 (to M. E. R.), R01 AI45898-01 (to M. E. R.) and the Human Frontier Science Program (to M. E. R. and P. S. F.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Web Figure 1.
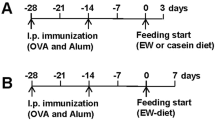
Experimental regime and oral antigen–induced TH2 immunity. (a) Mice were intraperitoneally (i.p.) injected with 50 μg OVA/1mg alum (OVA/Alum) on day 0. On days 12 and 15, mice were challenged orally with 20 mg placebo or OVA enteric-coated beads (placebo beads or OVA bead, respectively), or by intragastric administration (i.g.) of 200 μl PBS or soluble OVA (1 mg)–PBS. Four days after the last challenge, mice were killed and parameters were measured. (b) Eosinophil numbers in peripheral blood were determined by Discombe's analysis of femoral artery blood. (c) The serum allergen-specific IgG1and IgG2a ratio is shown for unchallenged and challenged mice. Amounts of allergen-specific IgA (c) and IgG1 (d) in fecal extracts of sensitized mice challenged with placebo or allergen beads are shown. (f-–g) Mesenteric lymph nodes from placebo- and oral allergen-–challenged mice were cultured for 72 h with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) or OVA (50 μg/ml). Culture supernatants were collected and assessed for IL-5 (f), IL-4 (g) and IFN-γ (h) by ELISA. Data represent mean±s.e.m. for groups of 4-–5 per group. (GIF 67 kb)
Web Figure 2.
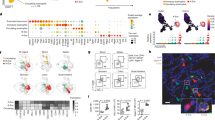
Anti-MBP immunostaining of the intestine in allergen-challenged mice. Allergen-sensitized mice were challenged with OVA enteric-coated beads. Four days later, the jejunum was analyzed. Anti-MBP staining shows intracellular granule and extracellular-associated immunoreactivity. (a,b) Two sections that highlight extracellular MBP localization are shown. (JPG 56 kb)
Rights and permissions
About this article
Cite this article
Hogan, S., Mishra, A., Brandt, E. et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol 2, 353–360 (2001). https://doi.org/10.1038/86365
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/86365
This article is cited by
-
Eosinophils in the pathogenesis of pancreatic disorders
Seminars in Immunopathology (2021)
-
Eosinophilic Gastritis/Gastroenteritis
Current Gastroenterology Reports (2021)
-
Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: a Randomized Double-blind Placebo Controlled Trial
Scientific Reports (2020)
-
The Immunologic Mechanisms of Eosinophilic Esophagitis
Current Allergy and Asthma Reports (2016)
-
Eosinophilic Gastroenteritis and Colitis: a Comprehensive Review
Clinical Reviews in Allergy & Immunology (2016)