Abstract
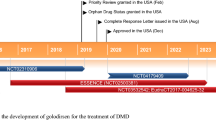
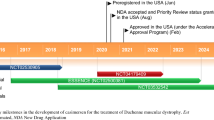
Nonsense mutations are implicated in 5–70 % of individual cases of most inherited diseases, including Duchenne muscular dystrophy (DMD) and cystic fibrosis. Ataluren (Translarna™) is an orally available, small molecule compound that targets nonsense mutations, and is the first drug in its class. Ataluren appears to allow cellular machinery to read through premature stop codons in mRNA, enabling the translation process to produce full-length, functional proteins. This article summarizes the milestones in the development of ataluren leading to its conditional first approval for nonsense mutation DMD.
Similar content being viewed by others
References
Kellermayer R. Translational readthrough induction of pathogenic nonsense mutations. Eur J Met Genet. 2006;49(6):445–50.
Mendell JT, Dietz HC. When the message goes awry: disease-producing mutations that influence mRNA content and performance. Cell. 2001;107(4):411–4.
PTC Therapeutics Inc. PTC therapeutics receives orphan drug designation for PTC124 for the treatment of cystic fibrosis [media release]. 2004. http://www.ptcbio.com.
PTC Therapeutics Inc. European Medicines Agency (EMEA) Grants PTC therapeutics orphan drug designation for PTC124 for the treatment of duchenne muscular dystrophy and cystic fibrosis [media release]. 2005. http://www.ptcbio.com.
PTC Therapeutics Inc. FDA’s office of orphan products development awards PTC therapeutics a grant to support clinical development of PTC124 for the treatment of nonsense-mutation-mediated cystic fibrosis [media release]. 2005. http://www.ptcbio.com.
PTC Therapeutics Inc. PTC therapeutics receives orphan drug designation for PTC124 for the treatment of duchenne muscular dystrophy [media release]. 2005. http://www.ptcbio.com.
PTC Therapeutics Inc. FDA awards PTC therapeutics orphan products development grant for the development of PTC124 in Duchenne muscular dystrophy [media release]. 2006. http://www.ptcbio.com.
PTC Therapeutics Inc. PTC therapeutics announces $25 million award from cystic fibrosis foundation therapeutics for development of PTC124 [media release]. 2008. http://www.ptcbio.com.
PTC Therapeutics Inc. PTC therapeutics announces european medicines agency validation of marketing authorization application for ataluren in Duchenne muscular dystrophy [media release]. 2012. http://ir.ptcbio.com/releasedetail.cfm?ReleaseID=725244.
PTC Therapeutics Inc. PTC therapeutics receives positive opinion from CHMP for Translarna™ (ataluren) [media release]. 2014. http://ir.ptcbio.com/releasedetail.cfm?ReleaseID=850246.
PTC Therapeutics Inc. PTC therapeutics provides update on CHMP opinion for conditional approval of ataluren for nonsense mutation Duchenne muscular dystrophy [media release]. 2014. http://ir.ptcbio.com/releasedetail.cfm?ReleaseID=820977.
PTC Therapeutics. PTC therapeutics receives conditional approval in the European Union for Translarna™ for the treatment of nonsense mutation Duchenne muscular dystrophy [media release]. 2014. http://ir.ptcbio.com/ReleaseDetail.cfm?ReleaseID=863914.
European Commission. Commission Immplemeting Decision of 31.7.14 granting a conditional marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of the Council for “Translarna – ataluren”, an orphan medicinal product for human use [media release]. 2014. http://ec.europa.eu/health/documents/community-register/2014/20140731129187/dec_129187_en.pdf.
PTC Therapeutics. PTC therapeutics reports first quarter financial results and provides corporate update [media release]. 2014. http://ir.ptcbio.com/releasedetail.cfm?ReleaseID=845413.
Genzyme Corporation, PTC Therapeutics Inc. Genzyme corporation and PTC therapeutics announce collaboration on small molecule for genetic diseases [media release]. 2008. http://www.genzyme.com.
Sanofi-Aventis. Sanofi-Aventis completes acquisition of genzyme corporation [media release]. 2011. http://www.sanofi-aventis.com.
PTC Therapeutics Inc, Genzyme. PTC therapeutics and genzyme announce restructuring of collaboration [media release]. 2011. http://www.ptcbio.com.
Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447(7140):87–91.
Li M, Andersson-Lendahl M, Sejersen T, et al. Muscle dysfunction and structural defects of dystrophin-null sapje mutant zebrafish larvae are rescued by ataluren treatment. FASEB J. 2014;28(4):1593–9.
Finkel RS, Flanigan KM, Wong B, et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLoS ONE. 2013;. doi:10.1371/journal.pone.0081302.
Du M, Liu X, Welch E, et al. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105(6):2064–9.
Kerem E, Hirawat S, Armoni S, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372(9640):719–27.
Sermet-Gaudelus I, De Boeck K, Casimir GJ, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med. 2010;182(10):1262–72.
Wilschanski M, Miller LL, Shoseyov D, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38(1):59–69.
Auld DS, Thorne N, Maguire WF, et al. Mechanism of PTC124 activity in cell-based luciferase assays of nonsense codon suppression. Proc Natl Acad Sci USA. 2009;106(9):3585–90.
McElroy SP, Nomura T, Torrie LS, et al. A lack of premature termination codon read-through efficacy of PTC124 (ataluren) in a diverse array of reporter assays. PLoS Biol. 2013;11(6):e1001593.
Goldmann T, Overlack N, Wolfrum U, et al. PTC124-mediated translational readthrough of a nonsense mutation causing usher syndrome type 1C. Hum Gene Ther. 2011;22(5):537–47.
Sarkar C, Zhang Z, Mukherjee AB. Stop codon read-through with PTC124 induces palmitoyl-protein thioesterase-1 activity, reduces thioester load and suppresses apoptosis in cultured cells from INCL patients. Mol Genet Metab. 2011;104(3):338–45.
Tan L, Narayan SB, Chen J, et al. PTC124 improves readthrough and increases enzymatic activity of the CPT1A R160X nonsense mutation. J Inherit Metab Dis. 2011;34(2):443–7.
Melhem M, Van Wart S, Rubino C, et al. Population pharmacokinetic (PK) analyses to bridge ataluren disposition between healthy adults and patients with nonsense mutation duchenne-becker muscular dystrophy (nmDBMD) [abstract no. W-023]. J Pharmacokinet Pharmacodyn. 2013;40(suppl. 1):S121–3.
Hirawat S, Welch EM, Elfring GL, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47(4):430–44.
Bushby K, Finkel R, Wong B, et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve. 2014. doi: 10.1002/mus.24332 (Epub ahead of print).
Kerem E, Konstan MW, De Boeck K, et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2(7):539–47.
PTC Therapeutics Limited. Translarna (ataluren) European Summary of Product Characteristics. 2014. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm408475.htm. Accessed 15 Aug 2014.
Disclosure
The preparation of this report was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. N. J. Ryan is a contracted employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Ryan, N.J. Ataluren: First Global Approval. Drugs 74, 1709–1714 (2014). https://doi.org/10.1007/s40265-014-0287-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0287-4