Abstract
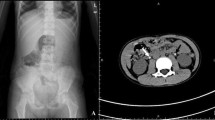
Urinary tract calculi have been reported to account for between 1 in 1,000 and 1 in 7,600 hospital admissions in children in the USA. The annual incidence of urolithiasis in patients older than 10 years is 109 per 100,000 of the population in men and 36 per 100,000 of the population in women in Minnesota. The use of various medications is considered to be one of the etiologic factors of nephrolithiasis. Ceftriaxone is a widely used third-generation cephalosporin that is generally considered very safe, but complications such as biliary pseudolithiasis, and rarely, nephrolithiasis have been reported in children. There is limited information about urolithiasis as a side effect of ceftriaxone. The aim of this study was evaluation of the incidence of nephrolithiasis following ceftriaxone therapy in children. This quasi-experimental before and after study was conducted in Mofid Children’s Hospital between 2003 and 2005. All patients were treated with 75 mg/kg intravenous ceftriaxone. Diagnosis of pyelonephritis was based on standard criteria. The first renal ultrasonography was performed on the first or second day of admission and was repeated on the last day of treatment. We also evaluated complicated patients for the third time with renal ultrasonography 3 months after treatment. Stone-forming patients underwent metabolic kidney stone risk factor evaluation. We evaluated 284 patients with pyelonephritis, 185 girls and 99 boys. The first ultrasonography was normal in all of our patients. On the second ultrasonography renal stones were reported in 4 out of 284 cases (1.4% and CI = 0.96–1.83%). Underlying metabolic risk factors could not be identified in stone-forming patients. Follow-up ultrasonography 3 months later was normal. The results of our study suggest that ceftriaxone-treated patients may be at an increased risk of kidney stone formation. Stones passed spontaneously in all affected patients so the use of this effective drug can be safely continued. Close monitoring of ceftriaxone-treated patients with regard to kidney stone formation is recommended.




Similar content being viewed by others
References
Milliner DS (2004) Calculi. In: Kaplan BS, Meyers KEC (eds) Pediatric nephrology and urology. Elsevier, Mosby, pp 361–373
Johnson CM, Wilson DM, O’Fallon WM (1979) Renal stone epidemiology: a 25-year study. Kidney Int 16(5):624–631
Matlaga BR, Shah OD, Assimos DG (2003) Drug-induced urinary calculi. Rev Urol 5(4):227–231
Avci Z, Koktener A, Uras N (2004) Nephrolithiasis associated with ceftriaxone therapy: a prospective study in 51 children. Arch Dis Child 89(11):1069–1072
Biner B, Oner N, Celtik C (2006) Ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound 34(5):217–222
Milliner DS (2004) Urolithiasis. In: Avner ED, Harmon WE, Niaudet P (eds) Pediatric nephrology. Lippincott Williams and Willkins, New York, pp 1091–1111
De Moor RA, Egberts AC, Schroder CH (1999) Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis. Eur J Pediatr 158(12):975–977
Cochat P, Cochat N, Jouvenet M (1990) Ceftriaxone-associated nephrolithiasis. Nephrol Dial Transplant 5(11):974–976
Prince JS, Senac MO Jr (2003) Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis in a child. Pediatr Radiol 33(9):648–651
Grasberger H, Otto B, Loeschke K (2000) Ceftriaxone-associated nephrolithiasis. Ann Pharmacother 34(9):1076–1077
Blais C, Duperval R (1994) Biliary pseudolithiasis in a child associated with 2 days ceftriaxone therapy. Pediatr Radiol 24(3):218–219
Park HZ, Lee SP, Schy AL (1991) Ceftriaxone-associated gallbladder sludge. Gastroenterology 100(6):1665–1670
Michielsen PP, Fierens H, Van Maercke YM (1992) Drug induced gallbladder disease. Incidence, aetiology and management. Drug Saf 7(1):32–45
Acknowledgements
The authors would like to thank the reviewers of this manuscript for their careful and thoughtful comments, and also the physicians, radiologists, and nurses of the Mofid Children’s Hospital and the Pediatric Infectious Research Center for their great assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohkam, M., Karimi, A., Gharib, A. et al. Ceftriaxone associated nephrolithiasis: a prospective study in 284 children. Pediatr Nephrol 22, 690–694 (2007). https://doi.org/10.1007/s00467-006-0401-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0401-2