Abstract
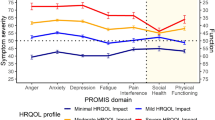
Health-related quality of life (HRQOL) is an important indicator of the burden of illness in moderate-to-severe plaque psoriasis. This study evaluated self-reported generic HRQOL among pediatric patients with moderate-to-severe plaque psoriasis based on pooled baseline clinical trial data and compared them to four common chronic diseases and to a healthy sample. The Pediatric Quality of Life Inventory Version 4.0 (PedsQL™ 4.0) Generic Core Scales was administered to 208 patients ages 4 to 17 years with stable, moderate-to-severe plaque psoriasis. Patients with moderate-to-severe plaque psoriasis were compared using one-sample t-tests to published PedsQL™ (http://www.pedsql.org) data on healthy children and pediatric patients with arthritis, psychiatric disorders, asthma, and diabetes. Pediatric patients with moderate-to-severe plaque psoriasis demonstrated significantly impaired physical, emotional, social, and school functioning in comparison to healthy children. The PedsQL™ Emotional and School Functioning Scales demonstrated the largest mean difference between the two groups (12.1, 11.1 points, respectively). In general, patients with plaque psoriasis demonstrated significantly more impaired generic HRQOL compared to patients with diabetes, comparable HRQOL to arthritis and asthma, and better HRQOL than psychiatric patients. In conclusion, the findings indicate that pediatric patients with moderate-to-severe plaque psoriasis have significantly impaired generic HRQOL in comparison to healthy children, and HRQOL generally comparable to other serious chronic diseases. These results demonstrate the significant negative impact of plaque psoriasis on the daily lives of these children from the patients’ perspective.
Similar content being viewed by others
Abbreviations
- PedsQL™:
-
Pediatric Quality of Life Inventory™
- FDA:
-
Food and Drug Administration
- HRQOL:
-
Health-related quality of life
- NS:
-
Nonsignificant
- BSA:
-
Body-surface area
- PASI:
-
Psoriasis area-and-severity index
- CI:
-
Confidence interval
References
Bastiaansen D, Koot HM, Bongers IL, Varni JW, Verhulst FC (2004) Measuring quality of life in children referred for psychiatric problems: psychometric properties of the PedsQL™ 4.0 Generic Core Scales. Qual Life Res 13:489–495
Bastiaansen D, Koot HM, Ferdinand RF, Verhulst FC (2004) Quality of life in children with psychiatric disorders: self, parent, and clinician report. J Am Acad Child Adolesc Psychiatry 43:221–230
Beattie PE, Lewis-Jones MS (2006) A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol 155:145–151
Clarke SA, Eiser C (2004) The measurement of health-related quality of life in pediatric clinical trials: a systematic review. Health Qual Life Outcomes 2(66):1–5
Cohen J (1988) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
de Jager MEA, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB (2010) A cross-sectional study using the Children’s Dermatology Life Quality Index (CDLQI) in childhood psoriasis: negative effect on quality of life and moderate correlation of CDLQI with severity scores. Br J Dermatol 163:1099–1101
Dillman DA, Smyth JD, Christian LM (2009) Internet, mail and mixed-mode surveys: the tailored design method. Wiley, New York
FDA (2009) Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Food and Drug Administration, U.S. Department of Health and Human Services, Rockville, MD
Fredriksson T, Pettersson U (1978) Severe psoriasis: oral therapy with a new retinoid. Dermatologica 157:238–244
Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ (2004) Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 51:704–708
Heydendael VMR, de Borgie CAJM, Spuls PI, Bossuyt PMM, Bos JD, de Rie MA (2004) The burden of psoriasis is not determined by disease severity only. J Invest Dermatol Symp Proc 9:131–135
Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y (2005) The psychosocial burden of psoriasis. Am J Clin Dermatol 6:383–392
Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T (1998) The impact of psoriasis on quality of life. Arch Dermatol 137:280–284
Langley RG, Paller AS, Hebert AA, Creamer K, Weng HH, Jahreis A, Globe D, Patel V, Orlow SJ (2011) Patient-reported outcomes in pediatric patients with psoriasis undergoing etanercept treatment: 12-week results from a phase III randomized controlled trial. J Am Acad Dermatol 64:64–70
Lewis-Jones MS, Finlay AY (1995) The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 132:942–949
Paller AS, Siegfried EC, Langley RG, Gottlieb AB, Pariser D, Landells I, Hebert AA, Eichenfield LF, Patel V, Creamer K, Jahreis A (2008) Etanercept treatment for children and adolescents with plaque psoriasis. New Engl J Med 358:241–251
Patrick DL, Deyo RA (1989) Generic and disease-specific measures in assessing health status and quality of life. Med Care 27:S217–S233
Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM (1999) Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 41:401–407
Revicki DA, Menter A, Feldman SR, Kimel M, Harnam N, Willian MK (2008) Adalimumab improves health-related quality of life in patients with moderate to severe plaque psoriasis compared with the United States general population norms: results from a randomized, controlled Phase III study. Health Qual Life Outcomes 6(75):1–8
Russo PA, Ilchef R, Cooper AJ (2004) Psychiatric morbidity in psoriasis: a review. Australasian J Dermatol 45:155–161
Sampogna F, Tabolli S, Soderfeldt B, Axtelius B, Aparo U, Abeni D (2006) Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol 154:844–849
Shikiar R, Willian MK, Okun MM, Thompson CS, Revicki DA (2006) The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes 4(17):1–12
Upton P, Lawford J, Eiser C (2008) Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res 17:895–913
Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL (2003) The PedsQL™ in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory™ Generic Core Scales and type 1 Diabetes Module. Diabetes Care 26:631–637
Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N (2004) The PedsQL™ in pediatric asthma: reliability and validity of the Pediatric Quality of Life Inventory™ Generic Core Scales and Asthma Module. J Behav Med 27:297–318
Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL™ 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 3:329–341
Varni JW, Limbers CA, Burwinkle TM (2007) How young can children reliably and validly self-report their health-related quality of life? An analysis of 8,591 children across age subgroups with the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes 5(1):1–13
Varni JW, Limbers CA, Burwinkle TM (2007) Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes 5(43):1–15
Varni JW, Seid M, Knight TS, Burwinkle TM, Brown J, Szer IS (2002) The PedsQL™ in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory™ Generic Core Scales and Rheumatology Module. Arthritis Rheum 46:714–725
Varni JW, Seid M, Kurtin PS (2001) PedsQL™ 4.0: reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 Generic Core Scales in healthy and patient populations. Med Care 39:800–812
Varni JW, Seid M, Rode CA (1999) The PedsQL™: measurement model for the Pediatric Quality of Life Inventory. Med Care 37:126–139
World Health Organization (1948) Constitution of the World Health Organization: basic document. World Health Organization, Geneva
Conflicts of interest
Funding: Financial support for the preparation of the manuscript was provided by Amgen. Dr. Varni has received compensation from Amgen as a consultant. Drs. Gandra, Harrison, Hooper, and Baumgartner are employees of Amgen. Dr. Globe is a former employee of Amgen and is currently employed by Allergan.
Competing Interests: Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the PedsQL™.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varni, J.W., Globe, D.R., Gandra, S.R. et al. Health-related quality of life of pediatric patients with moderate to severe plaque psoriasis: comparisons to four common chronic diseases. Eur J Pediatr 171, 485–492 (2012). https://doi.org/10.1007/s00431-011-1587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-011-1587-2