Abstract
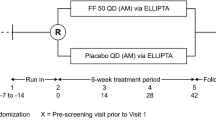
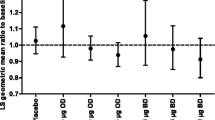
Fluticasone propionate is a synthetic steroid for use by the inhaled route. It's high topical potency and low systemic bioavailability make it suitable for use in asthmatic children. A total of 258 children were randomised in a double-blind study to receive fluticasone propionate (50 μg bd) as the dry powder formulation inhaled via a Diskhaler inhaler, or matched placebo (with current therapy) for 4 weeks throughout which time diary cards were completed. During clinic visits lung function and adrenal function were measured. Fluticasone propionate produced a significantly greater increase in morning peak expiratory flow rate (PEFR) (adjusted mean difference over days 1–28, 17 l/min (95% CI; 10, 24);P<0.001) and evening PEFR (adjusted mean difference over days 1–28, 16 l/min (95% CI; 9, 23);P<0.001). In addition, diary card symptom scores, beta2-agonist rescue and clinic lung function improved significantly on fluticasone propionate. There were few adverse events and basal plasma cortisol remained within the normal range. In conclusion fluticasone propionate at 50 μg bd is superior to placebo (current therapy) in the treatment of childhood asthma with no evidence of adverse effects.
Similar content being viewed by others
Abbreviations
- CI:
-
confidence intervals
- FEV1 :
-
forced expiratory volume in 1 s
- PEFR:
-
peak expiratory flow rate
References
Anderson HR (1992) Epidemiology of asthma Br J Hosp Med 47:99–104
Boe J, Skoogh B-E (1992) Is long-term treatment with inhaled steroids in adults hazardous? Eur J Respir Dis 5:1037–1039
Brown PH, Blundell G, Greening AP, Crompton GK (1991) Screening for hypothalamo-pituitary-adrenal axis suppression in asthmatics taking high dose inhaled corticosteroids. Respir Med 85:511–516
Geddes DM (1992) Inhaled corticosteroids; benefits and risks. Thorax 47:404–407
Gustaffson P, Tsanakas (1992) A double-blind study to compare the efficacy and safety of fluticasone propionate 200 μg daily (FP) with beclomethasone dipropionate 400 μg daily (BDP) in childhood asthma, both administered via a pressurised inhaler and spacer (abstract). Eur J Respir Dis 5 [Suppl 15]:1084
Harding SM (1990) The human pharmacology of fluticasone propionate. Respir Med 84 [Suppl A]:25–29
Harding SM, Felstead S (1988) A comparison of the tolerance and systemic effects of fluticasone propionate (FP) and beclomethasone dipropionate (BDP) in healthy volunteers (abstract). Eur J Respir Dis 1 [Suppl 2]:196S
Henrikson JM, Dahl R (1983) Effects of inhaled budesonide alone and in combination with low dose terbutaline in children with exercise-induced asthma. Am Rev Respir Dis 128:993–997
Lane DJ, Storr A (1980) Asthma the facts 1979. Oxford University press, Oxford
Phillips GH (1990) Structure-activity relationships of topically active steroids: the selection of fluticasone propionate. Respir Med 84 [Suppl A]:19–23
Polgar G, Promadhat V (1971) Pulmonary function testing in children: techniques and standards. WB Saunders, Philadelphia, pp 12–17
Svendsen UG (1990) Fluticasone propionate (a new inhaled steroid): clinical developments in mild to moderate adult asthmatics (Abstract). Eur J Respir Dis 3 [Suppl 10]:250s, S 924
Warner JO, Neijens HJ, Landau LI, Jones K, Ascher MJ, Rachelefsky GS, et al. (1992) Asthma: a follow-up statement from an international paediatric asthma consensus group. Arch Dis Child 67:240–248
Wolthers OD, Pedersen S (1992) Short term linear growth in children with asthma during treatment with inhaled fluticasone propionate and beclomethasone dipropionate (abstract) Eur J Respir Dis 5 [Suppl 15]:581
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
MacKenzie, C.A., Weinberg, E.G., Tabachnik, E. et al. A placebo controlled trial of fluticasone propionate in asthmatic children. Eur J Pediatr 152, 856–860 (1993). https://doi.org/10.1007/BF02073387
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02073387