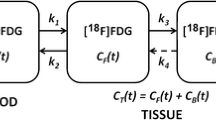
Summary
Sequential positron emission tomographic scans with [18F]-2-fluorodeoxyglucose (PET-FDG) were performed on 6 patients with glioblastoma multiforme who were treated with adjuvant BCNU. Scans were acquired before and 24 hours after BCNU. All patients had prior brain irradiation. Ratios between the maximal tumor FDG uptake and the contralateral white matter FDG uptake, the glucose uptake ratio, were determined. Percent changes in the glucose uptake ratio between the baseline scan and the 24 hour post-treatment scan were of prognostic significance. Patients with the largest percent changes in FDG uptake had the shortest survival. In contrast, neither the baseline glucose uptake ratio nor the visual tumor grade accurately predicted length of survival.
Similar content being viewed by others
References
Phelps ME, Huang SC, Hoffman EFet al.: Tomographic measurement of local cerebral glucose metabolic rate in Lumans with (F-18) 2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol 6: 371–388, 1979
Rhodes CG, Wise RJ, Gibbs JMet al.:In vivo disturbance of the oxidative metabolism of glucose in human cerebral gliomas. Ann Neurol 14: 614–626, 1983
Di Chiro G, DeLaPaz RL, Brooks RAet al.: Glucose utilization of cerebral gliomas measured by [18F] fluorode-oxyglucose and positron emission tomography. Neurology 32: 1323–1329, 1982
Di Chiro G, Brooks RA, Patronas NJ, Bairamian D, Kornblith PL, Smith BH, Mansi L, Barker J: Issues in thein vivo measurement of glucose metabolism of human central nervous system tumors. Ann Neurol 15 (suppl): S138-S146, 1984
Nelson T, Lucignani G, Goochee Jet al.: Invalidity of criticisms of the deoxyglucose method based on alleged glucose-6-phosphatase activity in brain. J Neurochem 46: 905–919, 1986
Weber G: Differential carbohydrate metabolism in tumor and host. In Arnott MS, van Eys J and Wang YM: Molecular interrelations of nutrition and cancer. Raven Press, New York, pp 191–208, 1982
Weber MJ, Salter DW, McNair TE: Increased glucose transport in malignant cells: Analysis of its molecular basis. Molecular interrelations of nutrition and cancer. Raven Press, New York, pp 183–190, 1982
Dominguez JE, Graham JF, Cummins CJet al.: Enzymes of glucose metabolism in cultured human gliomas: neoplasia is accompanied by altered hexokinase, phosphofructokinase, and glucose-6-phosphate dehydrogenase levels. Metab Brain Disease 2: 17–30, 1987
Loreck DJ, Galarraga J, Van der Feen J, Phang JM, Smith BH, Cummins CJ: Regulation of the pentose phosphate pathway in human astrocytes and gliomas. Metab Brain Disease 2: 31–46, 1987
Doyle WK, Budinger TF, Valk PEet al.: Differentiation of cerebral radiation necrosis from tumor recurrence by [18F] FDG and82Rb positron emission tomography. J Comput Assist Tomogr 11: 563–570, 1987
Valk PE, Budinger TF, Levin VA, Silver BA, Gutin PH, Doyle WK. PET of malignant cerebral tumors after interstitial brachytherapy. J Neurosurg 69: 830–838, 1988
DiChiro G, Oldfield E, Wright DCet al.: Cerebral Necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors. AJR 150: 189–197, 1988
Glantz MJ, Hoffman JM, Coleman REet al.: Identification of early recurrence of primary central nervous system tumors by [18F]fluorodeoxyglucose positron emission tomography. Ann Neurol 29: 347–355, 1991
Kushner M, Alavi A, Reivich Met al.: Contralateral cerebellar hypometabolism following cerebral insult: A positron emission tomography study. Ann Neurol 15: 425–434, 1984
Patronas NJ, Di Chiro G, Smith BHet al.: Depressed cerebellar glucose metabolism in supratentorial tumors. Brain Res 291: 93–101, 1984
Fukuyama H, Kameyama M, Harada Ket al.: Thalamic tumors invading the brain stem produce crossed cerebellar diaschisis demonstrated by PET. J Neurol Neurosurg Psychiatr 49: 524–528, 1986
Rozental JM, Levine RL, Nickles RJ, Dobkin JA, Hanson JM: Cerebral diaschisis in patients with malignant glioma. J Neuro-Oncol 8: 153–161, 1990
Alavi JB, Alavi A, Chawluk J, Kushner M, Powe J, Hickey W, Reivich M: Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 62: 1074–1078, 1988
Alavi JA, Alavi A, Goldberg HI, Dann R, Hickey W, Reivich M: Sequential computerized tomography and positron emission tomography studies in a patient with malignant glioma. Nuc Med Comm 8: 457–468, 1987
Patronas NJ, DiChiro G, Kufta C, Bairamian D, Kornblith PL, Simon R, Larson SM: Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg 62: 816–822, 1985
Rozental JM, Levine RL, Nickles RJ, Dobkin JA: Glucose uptake in gliomas after treatment: a positron emission tomographic study. Arch Neurol 46: 1302–1307, 1989
Rozental JM, Levine RL, Nickles RJ: Changes in glucose uptake by malignant gliomas: preliminary study of prognostic significance. J Neuro-Oncol 10: 75–83, 1991
Rozental JM, Levine RL, Nickles RJ, Dobkin JA: Acute change in glucose uptake by gliomas after chemotherapy. Ann Neurol 26: 182 (Abstr), 1989
Rozental JM, Levine RL, Dobkin JA, Nickles RJ: Acute changes in glioma metabolism after treatment. J Neuro-Oncol 7: S23 (Abstr), 1989
Rozental JM, Robins HI, Finlay Jet al.: Eight-drugs-in-one-day chemotherapy in postirradiated adult patients with malignant gliomas. Med Pediatr Oncol 17: 471–476, 1989
Rozental JM, Levine RL, Mehta MPet al.: Early changes in tumor metabolism after treatment: the effects of stereotactic radiotherapy. Int J Radiat Oncol, Biol, Phys 20: 1053–1060, 1991
Naruse S, Hirakawa K, Horikawa Y, Tanaka C, Higuchi T, Ueda S, Nishikawa H, Watari H: Measurements ofin vivo 31P nuclear magnetic resonance spectra in neuroectodermal tumors for evaluation of the effects of chemotherapy. Cancer Res 45: 2429–2433, 1985
Steen RG: Response of solid tumors to chemotherapy monitored byin vivo 31P nuclear magnetic resonance spectroscopy: a review. Cancer Res 49: 4075–4085, 1989
Steen RG, Tamargo RJ, McGovern KA, Sunder Rajan S, Brem H, Wehrle JP, Glickson JD:In vivo 31P nuclear magnetic resonance spectroscopy of subcutaneous 9L gliosarcoma: effects of tumor growth and treatment with 1, 3-bis (2-chloroethyl)-1-nitrosourea on tumor bioenergetics and histology. Cancer Res 1988; 48: 676–681.
Li S-J, Wehrle JP, Sunder Rajan S, Steen RG, Glickson JD, Hilton J: Response of radiation-induced fibrosarco-ma-1 in mice to cyclophosphamide monitored byin vivo 31P nuclear magnetic resonance spectroscopy. Cancer Res 48: 4736–4742, 1988
DiChiro G, Brooks RA: PET quantitation: blessing and curse. J Nucl Med 29: 1603–1604 (Editorial), 1988
Mazziotta JC: The continuing challenge of brain tumor management: the contribution of positron emission tomography. Ann Neurol 29: 345–346 (Editorial), 1991
Nelson JS, Tsukada Y, Schoenfeld D, Fulling K, Lamarch J, Peress N: Necrosis as a prognostic criterion in malignant supratentorial, astrocytic gliomas. Cancer 52: 550–554, 1983
Oken MH, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP: Toxicity and response criteria of the Eastern Oncology Cooperative Group. Am J Clin Oncol 5: 649–665, 1982
Rozental JM, Robins HI, Finlayet al.: Eight-drugs-in-one-day chemotherapy administered before and after radiotherapy to adult patients with malignant gliomas. Cancer 63: 2475–2481, 1989
Kim CK, Alavi JB, Alavi A, Reivich M: New grading system of cerebral gliomas using positron emission tomography with F-18 fluorodeoxyglucose. J Neuro-Oncol 10: 85–91, 1991
Brooks RA, DiChiro G, Zukerberg BW, Bairamian D, Larson SM: Test-retest studies of cerebral glucose metabolism using fluorine-18 deoxyglucose: validation of method. J Nucl Med 28: 53–59, 1987
Schefler WC: Statistics for Health professionals. Addison-Wesley Publishing Company, Reading, Massachussetts, pp 267–290, 1984
Langen KJ, Roosen N, Kuwert Tet al.: Early effects of intra-arterial chemotherapy in patients with brain tumours studied with PET: preliminary results. Nucl Med Comm 10: 779–790, 1989
Tyler JL, Diksic M, Villemure J-G, Evans AC, Meyer E, Yamamoto YL, Feindel W: Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med 28: 1123–1133, 1987
Jones SC, Alavi A, Christman Det al.: The radiation dosimetry of [F18] fluoro-2-deoxy-D-glucose in man. J Nucl Med 23: 613–617, 1982
Kokunai T, Tamaki N, Matsumoto S: Significance of DNA cross-links on 1-(4-amino-2-methylpyrimidin-5-yl)methyl-3-(2-chloroethyl)-3-nitrosourea (ACNU)-induced cytotoxicity against ACNU sensitive and -resistant lines of 9L rat glioma cells. JNCI 79: 119–122, 1987
Durbacz BW, Omidiji O, Gray DA, Shall S: (ADP-ri-bose)n participates in DNA excision repair. Nature 283: 593–596, 1980
Nduka N, Skidmore CJ, Shall S: The enhancement of cytotoxicity of N-methyl-N-nitrosourea and of gamma-radiation by inhibitors of poly(ADP-ribose) polymerase. Eur J Biochem 105: 525–530, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rozental, J.M., Cohen, J.D., Mehta, M.P. et al. Acute changes in glucose uptake after treatment: the effects of carmustine (BCNU) on human glioblastoma multiforme. J Neuro-Oncol 15, 57–66 (1993). https://doi.org/10.1007/BF01050264
Issue Date:
DOI: https://doi.org/10.1007/BF01050264