Article Text
Abstract
Objective To develop a clinical algorithm to identify paediatric patients who should be offered HIV testing in a setting of moderate HIV prevalence and limited resources.
Methods In a prospective cross-sectional study at Port Moresby General Hospital, Papua New Guinea, carers of inpatients were offered HIV testing and counselling for their children. Recruited children were tested for HIV antibodies and DNA. Standardised clinical information was collected. Multivariate regression analysis was used to ascertain independent predictors of HIV infection and these were used to develop a predictive algorithm.
Results From September 2007 to October 2008, 487 children were enrolled. Overall, 55 (11%) with a median age of 7 months were found to be HIV-infected. In multivariate analysis, independent predictors of HIV infection were: persistent fever (OR = 2.05 (95% CI 1.11 to 4.68)), lymphadenopathy (OR = 2.29 (1.12 to 4.68)), oral candidiasis (OR = 3.94 (2.17 to 7.14)) and being underweight for age (OR = 2.03 (1.03 to 3.99)). The presence of any one of these conditions had a sensitivity of 96% in detecting a child with HIV infection. Using an algorithm based on the presence of at least one of these conditions would result in around 40% of hospitalised children being offered testing.
Conclusions This clinical algorithm may be a useful screening tool for HIV infection in hospitalised children in situations where it is not feasible to offer universal HIV testing, providing guidance for HIV testing practices for increased identification and management of HIV-infected children in Papua New Guinea.
Statistics from Altmetric.com
Introduction
WHO guidelines for HIV testing recommend routine provider initiated HIV testing and counselling (PITC) for all patient groups including children presenting to hospital medical wards and outpatient facilities in countries experiencing generalised epidemics.1 Early diagnosis of HIV infection in children is important as it provides the pathway to appropriate care, treatment and support.2 3 Studies from resource limited settings report significant reductions in disease progression and mortality when HIV-infected infants and children receive cotrimoxazole preventive therapy (CPT) and antiretroviral therapy (ART).4,–,6
Papua New Guinea (PNG) is one of the worst affected countries in the Asia-Pacific region with an HIV prevalence estimated to be 2% in a population of roughly six million.7 An earlier retrospective study at Port Moresby General Hospital (PMGH) which is located in the capital city of PNG, indicated that only 4% of paediatric inpatients were being tested for HIV infection.8 The aim of this prospective study was to identify clinical conditions predictive of HIV infection, to develop an algorithmic clinical screening tool with a high sensitivity to help guide HIV testing at PMGH and to determine the likely impact of such a tool on HIV testing practice.
What is already known on this topic
▶ Difficulties in HIV diagnosis in the paediatric population represent a significant barrier to access to antiretroviral therapy in resource limited settings.
▶ Risk-based offering of testing in high prevalence, resource limited settings has been inadequate to prevent HIV-related mortality.
▶ The WHO has recently recommended provider initiated HIV testing and counselling for all patient groups in resource limited settings.
What this study adds
▶ Independent predictors of HIV infection in hospitalised children were: persistent fever, lymphadenopathy, oral candidiasis and being underweight for age.
▶ An algorithm based on the presence of at least one of these conditions would offer HIV testing to 40% of hospitalised children, with a sensitivity of 96% for detecting children with HIV infection.
▶ This clinical algorithm may be a useful screening tool for HIV infection in hospitalised children in situations where it is not feasible to offer universal HIV testing.
Methods
Participants were recruited from the PMGH paediatric wards. Exclusion criteria were previously confirmed HIV positive serostatus in children aged 2 years old or greater, or prior receipt of ART. Carers were approached by the study nurses between 8 am and 5 pm on weekdays. Recruitment days were based on the availability of the study nurses. The study was explained to the carers according to a written informed consent form and they were asked if they would like the children in their care to participate. If carers needed time to make a decision, the nurses returned to them later the same day or on the following day. In order to limit selection bias, testing was only offered to all patients who had been admitted within the preceding 24 h on a day of recruitment. Because of time constraints recruitment stopped once 10 children had been enrolled on a given day. Demographic and clinical data were collected using a standardised proforma.
All children were tested for HIV antibodies and for proviral DNA. Clinical history and blood samples were obtained by paediatric nurses who were also trained in counselling. If testing was refused following pretest counselling, children were withdrawn from further participation. Physical examination was carried out by the paediatric ward doctors. Information about the inpatient population from which the study population was drawn was collected from the ward record book containing data (age, sex, admission and discharge dates, and diagnoses) extracted by the ward clerks from the clinical notes after patients were discharged.
Serum samples were collected according to standard procedures at PMGH and tested locally for antibodies. These local serology tests were not used in the study analysis but provided a rapid result to guide and optimise immediate clinical care. Dried blood spot collection followed published guidelines.9 10 Samples were transported to Sydney, Australia and analysed for HIV antibodies using antibody ELISA Genetic Systems rLAV EIA HIV-1 enzyme immunoassay (Biorad, Marnes La Coquette, France) followed by western blot confirmation with Biorad New LAV Blot HIV-1 immunoblot (Biorad Marnes, La Coquette, France), and for proviral DNA using Amplicor HIV-1 test version 1.5 (Roche, USA).
Physical examination was standardised using definitions based mainly on the WHO/UNICEF Integrated Management of Childhood Illness clinical case definitions.11 Hepatomegaly and splenomegaly were further defined according to other published articles.12,–,14 The 2006 WHO Child Growth reference standards weight-for-age Z scores were used to define children as underweight for age.15
Data were analysed using STATA Release 10.0 (StataCorp LP, College Station, Texas, USA) and SPSS V.15.16 17
Univariate and multivariate logistic regression analyses were conducted on the whole study population to obtain p values, OR and 95% CI for variables predictive of HIV infection. First, the univariate associations between the independent variables and HIV status were analysed. For multivariate modelling, the same covariates were considered. Then the step-forward technique was used to reach the final model, in which factors with the smallest p value were added one at a time until all predictors in the model were significant at a p value of <0.05.
Once the final model was defined, an algorithm to identify children as having suspected symptomatic HIV infection based on the presence of one or more clinical conditions independently associated with HIV infection was constructed and evaluated by application to the study cohort in two ways. First, sensitivity, specificity and related measures were calculated as indicators of diagnostic properties in the full study population. Because this validation was based on the same population from which the algorithm was developed, a second validation was carried out using the split-sample method to develop a risk equation and internal validation.18 19 The study population was randomly allocated to a development sample set (50.7% of the population) and a validation sample set (49.3% of the population). Logistic regression was used to develop the prediction algorithm in the development set, and its accuracy was then assessed in the validation set using the same statistical methods described above for the full study population analysis.
Ethics approval for this study was granted by the Medical Research Advisory Committee, PNG, and the University of New South Wales Human Research Ethics Committee, Australia.
Results
During the study period from September 2007 to October 2008, 487 children were enrolled, representing 9% of all inpatients in the period. Information on age or gender was missing for seven and two children, respectively. The median age of the enrolled children was 11 months (range 1–135 months). Male children represented 53% of the study population. Fever and clinical signs consistent with pneumonia and malnutrition were the most common presenting clinical syndromes, occurring in approximately 35% of the study population.
Detailed clinical information was not collected from carers who had refused testing. However, admission diagnoses of the overall inpatient population in the ward record book were compared with selected clinical characteristics of the children recruited. The most common clinical symptoms and signs in the study population reflected the clinical symptoms and signs of the most common admission diagnoses of malnutrition, pneumonia and tuberculosis (TB).
A total of 55 (11%) had HIV infection. The median age for HIV-infected children was 7 months (range 1–80 months) and their gender distribution was equal. Information about maternal HIV status was collected for 388 women out of whom 88 were aware of their HIV status and of these 15 knew they were HIV positive. Ten HIV positive children were born to these women who were aware of their positive status.
Prediction of HIV infection in the full sample
On univariate analysis, there was a significant association between HIV infection and a clinical history of chest infection, persistent fever or chronic ear infection (table 1). Physical examination features associated with HIV infection on univariate analysis (table 2) were underweight for age, fever, wasting, lymphadenopathy, oral candidiasis and hepatomegaly. Multivariate regression analysis showed that four clinical features (persistent fever, lymphadenopathy, oral candidiasis and being underweight for age) were independently associated with HIV infection (tables 1 and 2).
Univariate and multivariate relationship between clinical history and HIV status for the study population
Univariate relationship between physical examination and HIV status for the study population
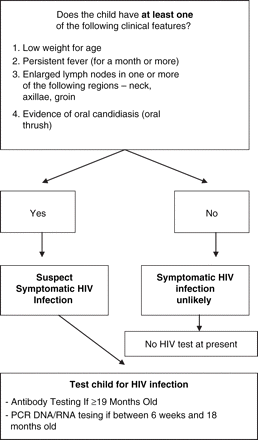
Based on these four conditions, the algorithm illustrated in figure 1 was defined to predict HIV infection. The sensitivity, specificity and positive predicitive value of these clinical conditions applied to the whole study population are shown in table 3. The proportion of children with HIV infection in the study population who were correctly identified as HIV-infected by different numbers of conditions is indicated in the last column of table 3.
Proposed algorithm.
Algorithm based on multivariate model applied to study population—sensitivity, specificity and PPV
The definitions used for the clinical conditions found to be associated with HIV infection on univariate and multivariate analysis are indicated in table 4. Other clinical definitions used in this study are detailed in appendix 1.
Definitions of clinical conditions associated with HIV infection in the study population
Split-sample analysis
The results of this analysis are published in the online version of this article.
Discussion
In this first prospective investigation of clinical predictors of HIV infection in children in PNG, nearly 500 children were successfully enrolled. The study population is not representative of the general paediatric population in PNG with the findings being applicable only to hospital settings. Difficulties in confirming diagnosis of common and important causes of morbidity such as TB and differentiation from pneumonia represent limitations despite the clinical case definitions used in the study. Furthermore, an age stratification analysis would have further delineated the clinical features predictive of HIV infection but was not feasible with our sample size. An additional limitation of this study is that the data set is a derived and not validated data set.
A definitive algorithm was chosen based on multivariate associations minimising the impact of correlations among clinical conditions. The ideal clinical algorithm to identify children with suspected HIV infection should have high sensitivity and specificity as well as being intuitive and easy to use. While specificity can be compromised to some extent, sensitivity should be as high as possible to minimise the number of children with HIV infection who might be missed.
The authors relied on the algorithm developed using multivariate regression analysis with the full study population as the definitive algorithm as it had acceptably high sensitivities and specificities. The circularity of developing and testing this algorithm in the same population is acknowledged. The authors were nevertheless encouraged by the similarity between the OR estimated in the split-sample analysis, and those used to develop the final algorithm.
A previously reported retrospective study,8 showed that approximately 4% of inpatients had been tested for HIV infection in a 12-month period in 2006; of these approximately 25% were found to be HIV-infected. In this prospective study, approximately 9% of inpatients were tested in a similar time period in 2007/2008 and 11% were HIV-infected. Consistent with findings from other resource limited settings,20,–,26 a number of clinical conditions were significantly associated with HIV infection including recent chest infection, persistent fever, ear infection, oral candidiasis, lymphadenopathy, hepatomegaly and malnutrition. Defining lymphadenopathy as two or more separate anatomical sites would have been more specific for generalised lymphadenopathy related to HIV. However to maximise the simplicity of data collection in this study it was defined as lymphadenopathy in any site. Chronic ear infection was not retained in the multivariate model most likely because it was found in only a small number (5%) of children in the study.
Considering an algorithm based on presentation with at least two of the designated clinical conditions, sensitivity and specificity were equal and moderate at 67%. The study population is representative of the ward population, and this would result in just over a quarter of admitted children being tested. With testing offered based on the presence of any one of the designated conditions according to the proposed algorithm (figure 1), the sensitivity of the algorithm increased to 96%, but the specificity dropped to 25%.
Previous studies have shown risk-based offering of testing to be inadequate to prevent HIV-related mortality in paediatric patients because of rapid disease progression and symptom overlap; these studies have been carried out in high prevalence, resource limited settings in Africa.27,–,31 There are inherent economic and logistical constraints on implementing a policy of routine testing in all resource limited settings including PNG. Routine testing of hospitalised children has been implemented successfully in African countries, for example, as recently reported in Lusaka, Zambia.32
The HIV prevalence of 11% recorded in this study supports an opt-out testing strategy as recommended by WHO. Additionally routine testing reduces associated stigma and increases acceptability of testing. It may be even less time consuming for healthcare workers, in some circumstances, to practice universal informed testing rather than selective testing based on clinical features. Despite the advantages of the opt-out testing approach, the resource implications of offering universal testing at PMGH remain a barrier to implementation. The algorithm developed in this study would allow the vast majority of cases of HIV infection to be detected by testing around 40% of admissions. This would represent a 10-fold increase in the proportion of paediatric inpatients tested at PMGH prior to this study.
A considerable proportion of HIV exposed but uninfected infants lose maternal HIV antibodies before the age of 9 months33 34. On this basis, rapid antibody testing has been used with success in countries such as Uganda to exclude HIV infection in infants, thereby reducing by 35% the need for HIV PCR testing.35 This approach was not explored in this study as the aim was to develop a simple screening tool based on clinical features rather than laboratory parameters (including the mother's HIV status) for guiding the decision about HIV testing.
Another strategy to screen maternally HIV exposed infants for a further DNA PCR test has been developed in Zimbabwe.36 This approach stratifies infants according to weight-for-age thresholds and uses this as a basis for deciding whether or not to carry out DNA PCR testing. While this strategy uses clinical features rather than a laboratory test and is notable for its simplicity, it would currently be of limited value in PNG as the findings from this study have indicated that the majority of women are unaware of their HIV status. Known maternal HIV infection was associated with a child being HIV positive, but the numbers of mothers that knew their HIV status was too small to allow comparison of features between those children with or without HIV positive mothers.
There has been progress in improving knowledge of maternal HIV status in PNG since the time of this study. The current national policy for antenatal clinics is for PITC with an ‘opt-out’ option. However, barriers to this process include access to antenatal clinics. While approximately 60% of women nationwide access antenatal care at least once during their pregnancy, the necessary tests may not be available in rural clinics and referral is difficult if a problem is discovered.37 Furthermore, of particular importance with respect to preventing mother-to-child transmission (PMTCT), only 38% of women in PNG have a professionally supervised birth37
Routine antenatal screening for HIV with provision of maternal ART, screening for TB, PMTCT, CPT for HIV exposed infants and early ART for HIV-infected infants remains the desirable integrated intervention that would dramatically reduce the burden of HIV-related morbidity and mortality in children. The clinical approach to identification of HIV infection as outlined in this study depends on the presentation of symptomatic disease. Clinical presentations such as oral candidiasis and wasting have been identified as independent risk factors for poor survival.38 This has negative implications for selective screening based on clinical criteria in that a child would have to have an immune system severely compromised by HIV infection to display recognisable symptoms and universal screening of all hospitalised children for HIV infection would, in contrast, most certainly identify asymptomatic children.
The authors have successfully developed a clinical algorithmic tool to guide PITC for hospitalised children. While universal screening as recommended by the WHO is preferable, an algorithmic screening tool to guide PITC in hospitalised children is relevant and useful in a resource limited setting with moderate HIV prevalence. This tool would increase testing of paediatric inpatients 10-fold compared to current practice and would facilitate earlier detection of HIV infection and earlier access to treatment for almost all HIV-infected children presenting to the facility.
Acknowledgments
The authors would like to thank the paediatric medical, nursing and clerical staff at PMGH for their assistance with this study.
References
Footnotes
-
Funding This study was funded by a National AIDS Council Secretariat (Papua New Guinea) Research Award. NCHECR is funded by the Australian Government Department of Health & Ageing.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the Medical Research Advisory Committee, Papua New Guinea, and the University of New South Wales Human Research Ethics Committee.
-
Provenance and peer review Not commissioned; externally peer reviewed.