Article Text
Abstract
Objective There is a lack of objective measures to assess children with acute wheezing episodes. Increased respiratory rate (RR) and pulsus paradoxus (PP) are recognised markers, but poorly recorded in practice. We examined whether they can be reliably assessed from a pulse oximeter plethysmogram (‘pleth’) trace and predict clinical outcome.
Patients and methods We studied 44 children aged 1–7 years attending hospital with acute wheeze, following initial ‘burst’ bronchodilator therapy (BT), and used custom software to measure RR and assess PP from oximeter pleth traces. Traces were examined for quality, and the accuracy of the RR measurement was validated against simultaneous respiratory inductive plethysmography (RIP). RR and PP at 1 hour after BT were compared with clinical outcomes.
Results RR from pleth and RIP showed excellent agreement, with a mean difference (RIP minus pleth) of −0.5 breaths per minute (limits of agreement −3.4 to +2.3). 52% of 1 min epochs contained 10 s or more of pleth artefact. At 1 hour after BT, children who subsequently required intravenous bronchodilators had significantly higher RR (median (IQR) 63 (62–66) vs 43 (37–51) breaths per minute) than those who did not, but their heart rate and oxygen saturation were similar. Children with RR ≥55 per minute spent longer in hospital: median (IQR) 30 (22–45) vs 10 (7–21) hours. All children who subsequently required hospital admission had PP-analogous pleth waveforms 1 hour after BT.
Conclusion RR can be reliably measured and PP detected from the pulse oximeter pleth trace in children with acute wheeze and both markers predict clinical outcome.
Trial registration number UKCRN15742.
- respiratory medicine
- paediatric emergency medicine
- technology
Data availability statement
Data are available upon reasonable request.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Respiratory rate and pulsus paradoxus are key indicators to identify severe exacerbations associated with acute wheezy episodes in preschool children.
Both respiratory rate and pulsus paradoxus are problematic to monitor in clinical practice and are therefore underutilised.
Analysis of the pulse oximeter plethysmogram trace enables accurate respiratory rate assessment in stable infants and children and can identify features analogous to pulsus paradoxus.
WHAT THIS STUDY ADDS
Recording accurate respiratory rate from the pulse oximeter plethysmogram is feasible in young children with acute wheezing in a busy emergency department.
In this setting, pulse oximeter plethysmogram analysis enables near-continuous monitoring of respiratory rate and of waveform features analogous to pulsus paradoxus.
Pulse oximetry-derived respiratory rate and pulsus paradoxus-analogous features predict response to treatment and outcome of children with wheezing in the emergency department.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This technique could be developed to permit real-time read-out of respiratory rate, in addition to oxygen saturation and heart rate, from a pulse oximeter.
Analysis of pulsus paradoxus-analogous features of the plethysmogram trace may provide a sensitive indicator of increased work of breathing.
Monitoring of these key vital signs may permit earlier recognition of a child who is deteriorating or failing to respond to treatment.
Introduction
Acute wheeze is a common clinical problem in young children, whether as an isolated viral-induced episode or in the context of diagnosed asthma.1 Assessing severity and response to treatment is particularly problematic in young children with lack of available objective measures.2 Respiratory rate (RR) is recognised as a key clinical sign in assessing respiratory disease,3–6 but in clinical practice, using visual counting, it is often poorly performed and inaccurate.7 8 Pulsus paradoxus (clinically detectable variation in pulse volume over the respiratory cycle) is a sign of increased work of breathing and a valuable marker of severe asthma exacerbations.6
Pulse oximetry is widely used to assess arterial oxygen saturation (SpO2) and also gives a pulse rate read-out from the plethysmograph (pleth) waveform. Respiration modulates the pulsatile pleth trace, resulting in baseline variation as well as pleth amplitude changes analogous to pulsus paradoxus.9 We have previously shown that a respiratory waveform extracted from the pleth by an appropriate low-pass filtering algorithm gives a reliable measure of RR in healthy newborns,10 preterm infants with chronic lung disease11 and young children with wheezing.12 We have also shown that RR can be derived from analysis of pleth amplitude variations analogous to pulsus paradoxus.10
When respiratory status deteriorates, RR tends to increase before SpO2 decreases.13 14 Thus continuous RR measurement could enable both earlier intervention and assessment of response to treatment. However, both RR and pulsus paradoxus assessments are normally measured intermittently and are prone to observer error. The aim of this study was to determine the feasibility, accuracy and clinical value of applying pleth-derived RR and pulsus paradoxus waveform assessment to young children attending a hospital emergency department (ED) with acute wheeze. We wished to determine whether (1) the technique could accurately measure RR compared with a gold standard technique (respiratory inductive plethysmography, RIP); (2) in a real-life acute clinical setting, a near-continuous measure of RR could be achieved; and (3) measuring RR could assess response to treatment and predict clinical outcome. We also explored whether (4) visible respiratory modulation of the pleth trace (RMP), analogous to clinical pulsus paradoxus, is associated with severity and outcome.
Patients and methods
The study was carried out at the ED of the Royal Alexandra Children’s Hospital, Brighton, UK. Children and parents were approached for informed consent as soon as practicable after arrival at the ED. Children were eligible if they were between 1 and 7 completed years of age, had a diagnosis of either acute asthma or viral-induced wheeze, and required ‘burst’ inhaled bronchodilator therapy (BT)—10 puffs of salbutamol every 20 min for an hour. Children with life-threatening features requiring immediate critical care were excluded.
Demographic data and details of wheezing history and treatment were collected. Clinical data, including heart rate (HR), RR by visual counting, arterial SpO2 measured by pulse oximeter and Clinical Asthma Score (CAS),15 were recorded 1 hour after completing the BT. As soon as possible after consent, a Nonin 3150 pulse oximeter was attached to a finger or toe and connected by Bluetooth to a nearby laptop computer, to which the pleth trace from the oximeter was recorded continuously using the Nonin OEM software. The signal was recorded as continuously as possible for up to 4 hours as tolerated.
Outcome data collected included need for intravenous bronchodilator treatment, requirement for admission to inpatient ward and to high dependency unit (HDU), total duration of stay in hospital, and duration of requiring hourly (or more frequent) inhaled bronchodilator therapy.
In addition, in a subset of children, chest and abdomen respiratory traces using RIP and pulse oximeter pleth trace from the Nonin device were recorded simultaneously with a Nonin oximeter pleth trace onto a SOMNOscreen Plus (SOMNOmedics, Germany) digital multichannel recorder for a 5 min period in order to validate the pleth measurement of RR.
HR, SpO2 and the pleth signal were exported to a spreadsheet file. HR, SpO2, pleth and RIP thoracic and abdominal band data acquired on the SOMNOscreen recorder were exported as .edf files. Both sets of data were imported into a custom software that we developed in MATLAB (The MathWorks, Natick, Massachusetts, USA). RIP RR was measured from the abdominal band trace.
RR from the oximeter pleth was derived as previously described.11 16 In summary, the pleth signal was low-pass-filtered using a filter with a cut-off frequency at half the median HR calculated over 1 min epochs; this filters out the HR output but leaves the RR component.12 The data were then visually analysed using a signal browser that displayed filtered pleth, raw pleth, SpO2, HR and RIP band data for the SOMNOscreen recordings. In addition to measuring the HR and RR from the pleth trace, we also calculated the HR to RR ratio, as our previous work suggested that this ratio is decreased during acute wheezy episodes—that is, RR increases disproportionately with HR.12
Traces from the subset of children who had simultaneous pulse oximeter and SOMNOscreen Plus recording were analysed for visible RMP—analogous to clinical pulsus paradoxus and defined as the presence of variations synchronous with respiration in both the pleth baseline and beat-to-beat pulse amplitude. Sections of the pleth trace with at least 20 s of regular respiratory pattern and little or no artefact were assessed for RMP by comparing with simultaneous RIP band traces using the software we developed with MATLAB (The MathWorks) to quantify and visualise pleth variability.
We compared the markers derived from the oximeter pleth analysis (RR, HR to RR ratio and RMP), as well as the HR and SpO2, all from pulse oximetry at 1 hour after BT, with the following clinical outcomes:
Subsequent need for hospital admission (and for admission to HDU).
Subsequent need for intravenous bronchodilator therapy.
Duration of hospital admission.
Duration of requiring at least hourly inhaled bronchodilators.
We defined children as significantly tachypnoeic if RR was 55 per minute or above, equivalent to the 99th centile of RR in hospitalised children in this age group.17
This was an exploratory study, but we carried out a prior power estimation based on our previous data on children attending with acute wheeze,12 indicating that the mean RR at presentation to the hospital was 49 (SD 13), and therefore that 54 children would be required to have 80% chance of demonstrating a mean difference of 10 breaths per minute between two clinical outcome groups. Data were analysed using Excel (Microsoft Corporation, USA) and Minitab V.19 (Minitab, USA). The Ryan-Joiner test in Minitab was applied to assess data distribution normality and parametric or non-parametric tests used as appropriate.
Results
There were 60 children screened for the study: 7 had mild acute wheeze and did not require BT; the remaining 53 were recruited after parental consent. Of these, four would not tolerate the pulse oximeter probe to remain in place, and in five recording could not be started (because parental consent had not been received) until more than 1 hour after completing BT.
Results are presented on the remaining 44 children (14 girls), with a median age of 41 (range 12–82) months. Of the children, 40 had suffered previous wheezing episodes and 17 were on regular therapy to prevent wheezing (inhaled corticosteroids or montelukast).
Validation of RR recording
These data are on a subset of 35 children, with a median age of 42 (range 12–82) months, in whom we were able to obtain a satisfactory 5 min simultaneous recording of pleth and RIP. Figure 1 shows a sample segment of recording.
Example of a 30 s duration section of recording with respiratory rate approximately 39 per minute. The ‘raw’ pleth trace shows baseline undulations synchronous with respiration. abdo, abdominal; pleth, plethysmogram.
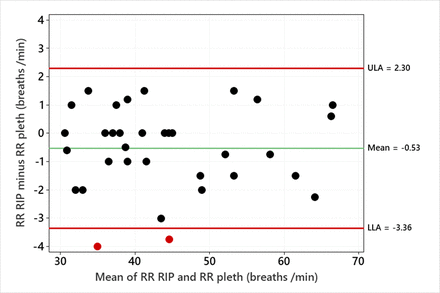
The mean RR from the abdominal RIP band traces was 44 (SD 10), ranging from 31 to 67 breaths per minute, and from the pleth was 44 (SD 10), ranging from 31 to 66 breaths per minute. The mean difference (RIP minus pleth) was −0.5 (SD 1.4) breaths per minute; the limits of agreement (RIP minus pleth) were −3.4 to +2.3 breaths per minute. Figure 2 shows a Bland-Altman plot of individual RR values from RIP and pleth.
Bland-Altman plot showing the difference between the RIP band and the filtered pleth-derived measurements of RR against the mean value. The two points in red are outside the limits of agreement, shown by the horizontal lines for the LLA and ULA. LLA, lower limit of agreement; pleth, plethysmogram; RIP, respiratory inductive plethysmography; RR, respiratory rate; ULA, upper limit of agreement.
Quality of pleth recordings
As shown in figure 1, when a good-quality pleth trace was available, the filtered pleth trace was almost identical in pattern and frequency to the RIP band traces. We have previously shown that this remains the case when respiration is irregular, with apnoeas.16 Artefact in more than a small proportion of the pleth trace (as with the RIP trace) can reduce the confidence in measuring RR. The presence of artefact in 20 pleth recordings from 43 to 125 min in length was assessed by visual inspection, dividing the traces into 1 min epochs. The mean proportion of 1 min epochs affected by 10 s or more of artefact was 52.3% (SD 19.4). We also looked at the maximum length of artefact-affected pleth recordings in the 11 children with 50% or more of epochs containing artefact. The median figure for the longest artefact-affected segment was 17 (range 7–31) min.
Prediction of clinical outcome
All 44 children were treated initially with BT. Four children subsequently required intravenous bronchodilator therapy (salbutamol, aminophylline or magnesium sulphate) due to poor response to inhaled therapy and all four were admitted to an HDU. Thirty-five children deemed improved and stable were allowed home directly from the ED, while nine children were admitted to an inpatient ward (including the four admitted to an HDU). Table 1 shows the mean (SD) clinical parameters 1 hour after the end of BT.
Summary of clinical parameters recorded 1 hour after completing burst bronchodilator therapy (BT)
The four children who later required intravenous bronchodilator therapy (and HDU admission) had significantly higher RR from pleth at 1 hour after BT (RRp1) than those who did not require intravenous therapy: median (IQR) 63 (62–66) vs 43 (37–51) breaths per minute (p=0.001, Mann-Whitney test). The HR to RR ratio at 1 hour after BT was lower in patients who required intravenous therapy compared with those who did not: median (IQR) 2.6 (2.6–2.7) vs 3.7 (3.2–4.2) (p=0.001, Mann-Whitney test). CAS was higher in those who required intravenous therapy: median (IQR) 9 (8.25–11.25) vs 7 (6.0–8.75). There was no significant difference in HR or SpO2 at 1 hour after BT between the two groups (for HR p=0.19, for SpO2 p=1.0, Mann-Whitney test). Figure 3 illustrates these markers graphically in relation to clinical outcome.
Individual value plots of RR (A), HR to RR ratio (B), HR (C) and SpO2 (D) against whether discharged from the ED, admitted to a general ward or admitted to HDU. Each point is plotted as a blue circle, with the yellow diamond symbols indicating the median value for each category. ED, emergency department; HDU, high dependency unit; HR, heart rate; pleth, plethysmogram; RR, respiratory rate; SpO2, oxygen saturation.
Children who had RRp1 of 55 per minute or above spent longer in hospital: median (IQR) 30 (22–45) vs 10 (7–21) hours (Mann-Whitney p=0.008). There was a non-significant trend for children with RRp1 55 per minute or above to have a longer duration of needing inhaled bronchodilator hourly or more frequently: median (IQR) 70 (60–146) vs 15.9 (57–490) min (Mann-Whitney p=0.38).
Respiratory modulation of the pleth trace
Pleth traces and RIP band recordings from 40 children with 5 min simultaneous pleth and RIP band recordings were evaluated. In nine children there was insufficient regular artefact-free respiratory trace to assess for RMP, so the results are presented for 31 children. Figure 4A illustrates a child showing clear variation with respiration both in pleth baseline and in beat-to-beat pleth amplitude variation. By contrast, there are no consistent baseline variations in pleth or beat-to-beat amplitude changes associated with RR in the child shown in figure 4B.
The 20 s duration sections of recording of pleth (pink upper trace) and RIP band (blue lower trace). For each graph, the centre trace (brown) shows the change in beat-to-beat amplitude of the pleth trace; each beat-to-beat amplitude point is shown with a circle marker. (A) The graph shows an example with clear baseline and pulse amplitude variation with respiratory rate. (B) The graph shows an example with no consistent baseline and pulse amplitude variation with respiratory rate from abdominal RIP band trace. pleth, plethysmogram; RIP, respiratory inductive plethysmography, pk, peak, min, minimum, amp, amplitude.
Nine children who had no clear RMP were all discharged from the ED, with a median time to discharge of 10.2 (IQR 6.9–16.0) hours. Twenty-two children with clear RMP had longer median time to discharge of 22.6 (IQR 8.9–30.0) hours (Mann-Whitney p=0.04), with eight requiring inpatient admission (four to HDU). RMP appeared most evident visually in periods of regular respiration.
Discussion
Clinical assessment of children with acute severe wheezing episodes is problematic and objective measures are needed. We have developed a technique for extracting a respiratory waveform from the pleth trace of a standard pulse oximeter. We have demonstrated that this approach yields an accurate estimate of RR and that a pleth trace of adequate quality can be obtained over long periods even in the challenging setting of unwell children in a busy ED. In this study we observed that the mean proportion of 1 min epochs affected by artefact (at least 10 s) was 52%. Previously, in stable, sleeping infants with chronic lung disease, we found that 20% of pleth epochs, but also 10% of RIP band epochs, were affected by artefact.11 It is not surprising that artefacts were more frequent in the older children in this study, who were awake, mobile and acutely unwell. Nevertheless, the availability of almost 50% of 1 min epochs artefact-free would allow near-continuous RR monitoring. Even in the most artefact-affected traces, the longest continuous affected period was 31 min.
Our results suggest that RR derived from the pleth trace can be used to assess response to treatment, specifically that children with persisting rapid RR after initial high-dose inhaled bronchodilator therapy are more likely to require admission to hospital, intravenous bronchodilator therapy and a longer hospital stay. Our results also suggest that absence of visible respiratory modulation of the pleth is associated with better outcomes. Although the presence of RMP (likely a sign of increased work of breathing) was associated with need for intravenous bronchodilator therapy, there was a large SD in the time to discharge; this suggests that RMP can also be seen in children who are not severely ill.
Tachypnoea is a recognised feature of acute severe asthma in adults, in whom RR correlates negatively with spirometric markers of airway obstruction.18 In children there is a lack of empirical evidence, but national guidelines encourage recording of RR and quote thresholds (without supporting data) to distinguish severe from moderate acute asthma.5 19 We are not aware of previous studies demonstrating an association between RR and outcomes in acute severe wheeze. This may be partly due to the difficulties and inaccuracies involved in clinical counting of RR.7
A potential criticism is that we used an arbitrary cut-off RR to identify those children who were significantly tachypnoeic after initial treatment and one which is different from the cut-offs described in clinical guidelines to define a severe attack.5 However, we based the cut-off value on normative data from large numbers of observations; these data17 20 have demonstrated that the normal RR values quoted in current guidelines are unrepresentative. The value of 55 breaths per minute is the midpoint of the 99th centile for RR for the lower (60 per minute) and upper (50 per minute) ends of our participants’ age range.17
Another potential criticism is that we may have included children with different pathophysiological conditions: younger children with viral-induced wheeze and older children with asthma. We recruited children broadly across the preschool years (over 12 months to under 7 years) and the nature of wheezing illness undoubtedly changes over this period. However, acute wheezing episodes remain predominantly viral-triggered throughout childhood,21 while categories of wheezing illness remain fluid over the preschool years.22
There have been previous attempts to use the pulse oximeter pleth trace to assess increased work of breathing in acute wheeze. Krishnan et al 23 recently reported that ‘visually detected pulsus paradoxus’ on the pleth trace at presentation to the ED had higher relative risks of adverse outcomes, although the authors’ definition of ‘pulsus paradoxus’ was unclear.24 Previous studies examining pulsus paradoxus-related phenomena from the pleth trace in acute wheeze have shown some association with lung function,25 26 but no clear correlation with clinical outcome.27 We observed that RMP was most evident in periods of regular respiration, which suggests that respiratory pattern should be considered when making such assessments. Our results are in keeping with those of Krishnan et al 23; in this study we clearly defined the phenomenon of visible RMP, analogous to clinical pulsus paradoxus. However, further work is needed to refine and validate a measure of the amount of RMP trace and to demonstrate that RMP can be reliably detected using the pleth trace alone.
The technique we have described has potential to improve the management of children with acute wheezing by providing near-continuous monitoring of a key physiological measurement (RR), which may become elevated before there is a fall in SpO2, without the need for an additional measuring device on the child. Clearly further development work is needed to allow the RR calculation to be performed in real time and to provide real-time quality control for artefact, but both these should be readily achievable with appropriate software. In addition to these technical considerations, caution is needed before advocating widespread introduction of continuous RR monitoring: in infants with bronchiolitis, continuous SpO2 monitoring has not been shown to improve clinical outcomes compared with intermittent SpO2 monitoring28 and over-reliance on SpO2 measurements in bronchiolitis may lead to increased hospital admissions.29 Larger clinical studies are needed to determine whether pleth-derived monitoring of RR and of pulsus paradoxus equivalents is effective in guiding decision-making in a child with acute wheezing.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the NHS Health Research Authority: London - Bloomsbury Research Ethics Committee (REC reference ID number: 13/LO/1427). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We are grateful for the assistance received from the staff of the Children’s Emergency Department at Royal Alexandra Children’s Hospital, Brighton.
References
Footnotes
Twitter @DWertheim
Deceased CO tragically died in 2019
Contributors DW and PCS conceptualised and designed the study, carried out the analysis of data, drafted the initial manuscript, and reviewed and revised the manuscript. CO and SLVLeM designed the data collection instruments, collected the data and carried out the initial analyses. OA carried out further data analysis and contributed to writing the manuscript. CO tragically died in 2019. All other authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. PCS takes overall responsibility as guarantor for the work.
Funding This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) programme (grant reference number PB-PG-0610-22433).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue