Article Text
Abstract
Objective: To characterise the demographics and aetiology of sudden cardiac death (SCD) in athletes referred to a tertiary cardiac pathology centre in the UK.
Design: Retrospective non-case controlled analysis.
Setting: Cardiac pathology centre at the National Heart and Lung Institute and Royal Brompton Hospital.
Subjects: Between 1996 and 2008, the hearts of 118 athletes were referred for pathological assessment to ascertain the precise aetiology of SCD.
Results: The majority of athletes (n = 113; 96%) were male and most (107; 91%) were amateurs participating predominantly in football, rugby and running. The mean (SD) age of death was 28 (12) years (range 7–59); 75% athletes were aged ⩽35 years. Most deaths (81%) occurred during or immediately after exercise. Antecedent symptoms of cardiac disease were reported in 21 (18%) subjects, and 20 (17%) had a family history of premature cardiovascular disease and/or SCD. 25 (21%) athletes had relevant past medical history which included a known history of cardiac disease. Cardiomyopathy was the commonest cause of death and accounted for 62% of all the SCDs. A significantly high proportion of athletes (23%) exhibited a morphologically normal heart. Atherosclerotic coronary disease accounted for only 3% of cases and was confined to athletes aged >35 years.
Conclusions: SCD in sport is largely due to clinically silent cardiomyopathies or primary electrical disorders (morphologically normal heart). Antecedent symptoms and family history are absent in over 80% of cases, and therefore clinical screening with health questionnaires will fail to identify most athletes with potentially sinister cardiac disorders.
Statistics from Altmetric.com
The sudden death of an athlete is a rare event with an incidence ranging between 1:50 0001 to 1:200 0002 a year. However, such events are highly publicised and have a substantial emotional impact on the community at large when one considers that athletes are perceived as the healthiest segment of society.
Over 80% of non-traumatic-exercise related deaths are attributable to cardiac disorders.1,2,3,4,5 The majority of sudden cardiac deaths (SCDs) in young athletes (<35 years) are due to hereditary or congenital cardiac anomalies; hypertrophic cardiomyopathy (HCM) is reported to be the commonest cause of death in young athletes world wide. In contrast, the vast majority of SCDs in older athletes are secondary to atherosclerotic coronary artery disease.
Most datasets examining SCD in athletes are derived from American3,5 and Italian6,7 studies. The United Kingdom lacks a national registry for systematically reporting sudden death in sports, and therefore knowledge relating to antecedent warning symptoms, the demographics of victims, circumstances and prevalent causes of sudden death in athletes in a sizeable cohort is lacking. However, such information is fundamental to facilitate any debate about local provisions for a potential pre-participation cardiovascular screening programme and subsequent exercise recommendations.8,9,10
The aim of this study was to identify the characteristics and causes of death in a large cohort of athletes referred to the Royal Brompton Hospital, a specialist tertiary cardiac pathology centre in the UK.
Methods
Between January 1996 and July 2008, 118 cases of sudden death in people participating in regular sport activities were referred to the Cardiac Risk in the Young (CRY) Centre for Cardiac Pathology at the Royal Brompton Hospital for further evaluation, by coroners and pathologists throughout the UK.
Definitions
The term “sudden death” was defined as sudden unexpected death (within 1–12 h of apparent wellbeing) from natural causes during or shortly after (within 24 h) exercise. The individual was considered an “athlete” if he or she participated in at least 2 h/week of organised physical training and competed in regular team or individual sport.
Subjects
Subjects were divided into two groups based upon their age at death: (a) ⩽35 years and (b) >35 years. Data on age, sex, circumstances of death, sporting discipline, antecedent cardiac symptoms, past medical history and a family history of cardiac disease (when available) of the deceased subject were obtained from the referring pathologist or coroner.
Toxicology screen
All patients included in this study underwent a toxicology screen as part of the coroner’s mandate since all deaths were sudden and unexpected.
Pathological analysis
Pathological analysis of all hearts was performed by the senior author (MNS) with the consent of the coroner and family of the deceased. The heart weight was recorded and measurements of the left and right ventricular wall thickness and internal cavity dimensions were made at mid-ventricular level excluding papillary muscle and fat. Sections of myocardium were fixed in formalin, embedded in paraffin and stained with haematoxylin and eosin as well as elastic Van Gieson stain to highlight myocardial fibrosis.
The extramural coronary arteries were studied macroscopically in the intact heart by making multiple cross sections of the vessels (3–5 mm apart). Table 1 gives the macroscopic and histological criteria for specific cardiac diseases.
Macroscopic and microscopic criteria for determining cardiac pathology
Results were reported in four broad categories: (a) cardiomyopathies; (b) coronary artery pathology; (c) morphologically normal heart (d) and other cardiac pathology.
Statistical analysis
Means and standard deviations (SD) were calculated for continuous variables. Data were compared with Student’s t test where appropriate.
Results
Demographics
Of the 118 cases of SCD, the majority were amateur sportsmen (n = 107, 91%) and included seven subjects who had participated in 2–23 marathons. The remaining 11 cases (9%) were seven athletes (6%) at a professional or semiprofessional level (soccer n = 6, cycling n = 1) and four (3%) who participated in intensive physical training in the armed forces.
The subjects were predominately male (n = 113; 96%). One hundred and thirteen athletes (96%) were white and five were black (African/Caribbean in origin). The mean (SD) age of SCD in this series was 27.9 (12.5) years (range 7–59). Seventy-five per cent of all deaths were in subjects aged ⩽35 years and almost one-third were in child or adolescent athletes (<18 years). The greatest number of deaths (n = 20) occurred in the 16–20 year age group (fig 1). With the exception of one case, all female athletes who died were in the younger age group.
Bar chart showing the number of sudden deaths in athletes in relation to age in 118 deaths in sportsmen referred to a tertiary centre in the UK over a 12-year period.
The vast majority of SCDs (81%) occurred during (66%) or immediately after (15%) exercise. In relation to sporting discipline, most deaths occurred in soccer (n = 44; 37%; age 11–35 years), followed by running (n = 24; 20%; age 8–59 years) and rugby (n = 11 cases; 9%, age 15–42 years) (table 2).
Demographic characteristics of the cohort
Antecedent symptoms, past medical history and family history of cardiac disease
Of the 118 cases, 21 (18%), had experienced one of more antecedent symptoms suggestive of underlying cardiac disease (table 3).
Antecedent symptoms, family history and relevant cardiac history of the 118 subjects*
In 20 subjects (17%) there was a family history of premature cardiovascular disease (with the majority of cases comprising ischaemic heart disease, 69%) and/or family history of SCD (⩽50 years old) in a first-degree relative.
Twenty-five (21%) of the subjects had relevant previous medical history. Seven subjects had a history of cardiac disease, nine were clinically suspected to have underlying cardiac pathology, five had risk factors for coronary artery disease and four had had at least one epileptic seizure (table 3). Ten athletes (8%) also had a previous diagnosis of asthma. As far as the authors can ascertain, none of the asymptomatic subjects had been subject to pre-participation cardiovascular evaluation to identify disorders capable of causing SCD.
Causes of sudden cardiac death
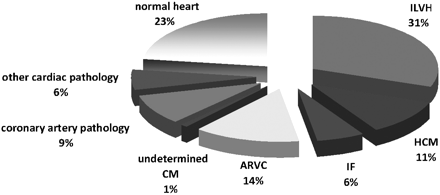
Abnormal cardiac pathology (macroscopic and/or microscopic) was identified in 91 (77%) of all cases. The remaining subjects had a morphologically normal heart (table 4 and fig 2).
Pie chart showing the causes of sudden cardiac death in 118 sports deaths referred to a tertiary cardiac centre in the UK over 12 years. ARVC, arrhythmogenic right ventricular cardiomyopathy; CM, cardiomyopathy; HCM, hypertrophic cardiomyopathy; IF, idiopathic fibrosis; ILVH, idiopathic left ventricular hypertrophy. Percentages do not add up to 100% because of rounding.
Cause of sudden cardiac death according to histopathological findings
Toxicology screen
All but two subjects had a normal toxicology screen. Both were body builders and had blood traces of anabolic steroids.
Cardiomyopathy
Deaths attributed to a primary myocardial disorder (cardiomyopathy) were identified in 73 (62%) of all athletes with an SCD. Left ventricular hypertrophy (LVH) was the most commonly identified abnormality on macroscopic examination and was detected in 49 (42%) athletes raising the possibility of HCM. However, only 13/49 (27%) athletes with LVH exhibited associated myocyte disarray, the historically regarded histological hallmark of HCM. The remaining cases of LVH (n = 36; 31%) were associated with histological evidence of either isolated myocyte hypertrophy (n = 27) or myocyte hypertrophy and fibrosis (n = 9). The authors classified these cases under the term “idiopathic LVH”. One case of HCM and another of idiopathic LVH was associated with the presence of anabolic steroid traces on toxicology screen.
Athletes with HCM were predominantly in the younger age group (mean (SD) age 24.6 (7.1); range 11–43) and all but one case was male. Similarly, 71% of all victims with idiopathic LVH were in the younger age group (mean (SD) age 32.7 (12.6) years; range 9–59) and all were male. Of the five Afro-Caribbean subjects, four had evidence of idiopathic LVH.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) was the second most common diagnosis and was identified in 16 (14%) members of our cohort. There was evidence of biventricular involvement in 50% of cases. In contrast with cases of idiopathic LVH and HCM, deaths were equally distributed between the older and younger age group (mean (SD) age 35.9 (12.2) years; range 16–57).
Idiopathic fibrosis without LVH occurred in seven cases with all but one in the younger age group (mean (SD) age 28.4 (8.5) years; range 17–43). Finally, one case exhibited features of both arrhythmogenic right ventricular cardiomyopathy and HCM. There was insufficient tissue sampling to provide a definite diagnosis and the case was classified as an undetermined cardiomyopathy.
Coronary artery pathology
Coronary artery pathology was identified in 11 (9%) subjects. The main pathology was a congenital anomaly of the coronary arteries which was seen in six of the 11 subjects all of whom were male and ⩽35 years old (mean (SD) age 15.8 (6.2) years; range 7–25). Both coronary arteries arose from the same coronary ostium in four cases; two cases had the left coronary artery arising from the right sinus and two cases had the right coronary artery arising from the left sinus. Of the remaining two cases, one had atresia and hypoplasia of the left coronary artery and the other stenosis of the ostium and a shelf-like slit of the left coronary artery. Coronary atherosclerosis was the cause of death in only three athletes who were all aged >35 years (mean (SD) age 49.7 (4.0) years; range 45–52). A single case of coronary artery spasm was detected in a 17-year-old man, as well as one case of spontaneous dissection of the left anterior descending artery in a 38-year-old man.
Other cardiac pathology
Lymphocyte myocarditis was the predominant finding in three cases. Two subjects had valvular disease which included floppy mitral valve and associated myocardial fibrosis. There was a single subject with corrected, complex congenital heart disease of univentricular circulation and Fontan circulation who exhibited biventricular hypertrophy with no other associated anomalies. Finally, one subject had evidence of an acute sickle cell crisis with sickling within the coronary arteries and associated acute ischaemia.
Morphologically normal heart
In almost a quarter of our cohort (23%) the post mortem disclosed no cardiac abnormality which could account for the cause of death, despite detailed macroscopic and microscopic examination. The majority of these cases (96%) were in the younger age group where the mean (SD) age of 18 (6.1) years (range 8–42) was significantly lower than that of those dying with identifiable cardiac pathology (p<0.001). Interestingly, three of the five swimmers who died had a morphologically normal heart.
Female athletes
Of the five females athletes in the series, two had a morphologically normal heart, one had HCM, one exhibited idiopathic fibrosis and another, coronary atherosclerosis.
Discussion
The cardiovascular benefits of regular physical activity are well established11 and only a small proportion of athletes with unsuspected cardiac pathology are at increased risk of exercise-related SCD.1,2,3,4,5,6,7 The majority of data examining the aetiology of deaths in athletes originates from the USA (1866 cases (whole series), 690 cases are primary CVD only)5 and Italy (55 cases),7 although a number of small studies also exist in other European countries, including Spain (61 cases),12 France (80 cases)13 and Ireland (51 cases).14 Data in the UK are scarce and limited to a small group of older sport participants.14,15,16 As far as we know, this study of 118 SCDs in athletes is the largest reported series in the UK.
Sport and gender predilection
Consistent with large American5,17 and Italian6,7 series of SCD in athletes, just over 80% of deaths occurred during or immediately after exercise, indicating that the interplay of physical, metabolic and endocrine stresses of exercise on the heart is an important trigger for fatal ventricular arrhythmias. Soccer and running were the sports most commonly associated with SCD and this is in agreement with most other studies.1,2,3,4,5,6,7,10 This sport bias most certainly represents the high participation rates in these sporting disciplines in most Western European countries. In concurrence with previous studies, the great majority (96%) of subjects with SCD subjects in our cohort were male.1,2,3,4,5,6,7,12,13,14,15,16,17 This may be attributed to the lower participation rate of women in sport generally and specifically in sports popular with male subjects, such as soccer, which is the predominant sport in our study.
Cardiomyopathy
Consistent with previous studies in the USA2,3,5,17 and Italy,1,6,7 our results indicate that cardiomyopathies are the most prevalent underlying pathology in SCD related to athletic activity. In contrast, however, in this series LVH without myocyte disarray, was the predominant finding (31%), compared with HCM and ARVC in the USA and Italy, respectively.
Idiopathic LVH is becoming increasingly recognised and although it has been reported in previous studies, this is the first series in which it predominates. It is unclear at this stage whether it represents an acquired pathological variant of the physiological LVH exhibited as part of the “athlete’s heart” in certain genetically predisposed backgrounds.18 The finding of idiopathic LVH in four out of five Afro-Caribbean cases of SCD may be relevant in this regard since a recent study in highly trained black athletes has shown that 3% exhibit substantial LVH (⩾15 mm) and it is plausible that in such athletes, marked LVH predisposes to exercise-related fatal ventricular arrhythmias.19 LVH has also been associated with the use of anabolic steroids.20 In our study traces of anabolic steroids were identified at post mortem in two body builders; one was diagnosed with idiopathic LVH and the other with HCM.
Idiopathic myocardial fibrosis with or without LVH, featured in 14% of this cohort in contrast to significantly lower rates in previous studies (2%–3%).12,13,14 The aetiology and importance of cardiac fibrosis remains unclear; however, transient myocardial damage has been detected in athletes in the post-race setting and has been associated with transient diastolic and systolic dysfunction.21 Possibly, in some athletes prolonged arduous physical activity may result in myocardial necrosis and subsequent fibrosis. This pathology may represent an acquired, exercise-related cardiomyopathy and/or genetic predisposition leading to a fatal arrhythmia. This concern was raised in a recent case report from our group, a marathon runner who died during a race with marked LVH and myocardial fibrosis.22 It is also possible that at least some of these cases may be due to the recently recognised familial arrhythmogenic left ventricular cardiomyopathy.23
ARVC was the second most common cardiomyopathy and accounted for 14% of all sudden deaths in our series. Our figures were significantly higher than those reported in the US series2,3,5,17 and not dissimilar from the reports from the Veneto region of Italy1,6,7,24 and other European countries.12,13,25,26,27,28 These observations suggest that there may be a higher genetic cluster of ARVC in Europe or, owing to the Venetian experience, a greater awareness of this disorder amongst pathologists in Europe.
Of note, we did not observe deaths from dilated cardiomyopathy in our cohort, which contrasts with series from other countries where the range was 2–11%.1,3,12,13
Coronary artery pathology
Coronary artery pathology was less prevalent in this study than in the US and Italian experience. Sudden cardiac death secondary to anomalous coronary arteries was confined to the younger age group (median 17 years) in this series, a trend supported by previous reports.2,3,5,6,7,12,13,17 Atherosclerotic coronary artery disease was seen in a much smaller number of patients and was confined to the older (>35 years) age group as observed in previous sports deaths series.14,15,16
Morphologically normal heart
In this study there was a high prevalence of SCDs in subjects with a morphologically normal heart (23%) despite detailed macroscopic and histological examination. Previous studies have reported significantly lower rates, as low as 1%,1,3,5,17 with only a Spanish series reporting a figure comparable to our study (16.3%).12 The identification of a morphologically normal heart is of great importance since studies in the USA and the UK suggest that more than 50% of such SCDs are caused by malignant arrhythmias secondary to the presence of inherited ion channel disorders usually affecting the potassium, sodium and calcium ion channels of the myocardial cells.29,30 A previous study undertaken at our unit highlights the prominent role of electrical abnormalities in the normal heart in SCD, particularly in younger subjects.31
Although selection bias has certainly contributed to the high prevalence of SCD with a morphologically normal heart, this study highlights the importance of establishing such a diagnosis since ion channelopathies can be identified in other relatives with non-invasive cardiac investigation, and appropriate treatment instigated to avoid further tragedies.32 Of interest, three children (aged 7–15 years) with morphologically normal hearts died during swimming. This sport has been particularly associated with deaths in the long Q–T syndrome33,34 and catecholaminergic polymorphic ventricular tachycardia.35
Clinical implications related to pre-participation screening
Sudden death is often the first clinical manifestation of underlying heart disease in young athletes. The Italian model of pre-participation cardiovascular screening in young athletes, using 12-lead ECG as a screening tool, is effective in reducing SCD by identifying athletes with underlying cardiomyopathy and ion channelopathies.6,7 This study indicates that based on the high prevalence of cardiomyopathies and the relatively low occurrence of coronary artery pathology in our subjects, pre-participation cardiovascular screening using a 12-lead ECG would probably have detected the underlying cardiac abnormality in a significant number of SCD victims. This argument is further reinforced by the high prevalence of SCDs in people with a morphologically normal heart, of which a significant proportion may be attributed to inherited ion channelopathies and which potentially might be detected by the 12-lead ECG.
The absence of antecedent cardiac symptoms and/or a family history of cardiac disease and/or SCD in almost 80% of cases of SCD confirms the prior observation that most cardiovascular disorders responsible for SCD in the athlete are clinically silent and unlikely to be discovered from spontaneous symptoms. Cardiovascular screening including ECG testing will be associated with a significantly higher diagnostic yield than reliance on history alone.28,36,37,38
We recognise that almost one-third of the cases in this series exhibited idiopathic LVH and it may argued that these cases may not have been identified with ECG. However, it is unclear at this stage whether idiopathic LVH represents a spectrum of the HCM phenotype, an exaggeration of the physiological response18 resulting in pathological LVH or whether it is indeed an innocent bystander (genuine physiological LVH) in a person who may have succumbed to a fatal ventricular disorder owing to an undetectable ion channel disorder or congenital accessory pathway. However, it is well established that over 90% of people with HCM have an abnormal ECG.39 The experience of two of the authors (SS and GW) of screening over 3000 highly trained British athletes aged 14–35 years identified three athletes who might have been considered to have HCM; all three exhibited grossly abnormal ECGs.40 Although ion channel disorders cannot be identified and accessory pathways may be difficult to demonstrate at autopsy, ante mortem 12-lead ECG has a high yield in the identification of ion channel disorders32 and accessory pathways. Based on these facts we suspect that a significant proportion of athletes with idiopathic LVH would have been identified on a routine ECG and in an expert setting would have been subject to further investigations aimed at confirming the pathological diagnosis and the assessment of risk of SCD.
The predominance of deaths due to idiopathic LVH and idiopathic myocardial fibrosis does raises the possibility that some deaths in sport may be secondary to acquired myocardial disorders resulting from the long-term effects of intensive exercise,18 warranting several cardiac assessments throughout the athlete’s career.
The Italian screening programme in athletes has been successful in identifying and preventing deaths predominantly from the cardiomyopathies through subsequent disqualification of the affected subject from sporting activities of moderate to high intensity to minimise the risk of SCD. In this regard it is prudent to highlight that in this study almost one-fifth of all SCDs in athletes occurred at rest, suggesting that the identification of cardiovascular diseases and subsequent disqualification from sport will not prevent deaths in all athletes harbouring potentially fatal cardiac disorders.
Limitations
We concede that conclusions drawn from this study do not necessarily apply to the whole of the UK because of a significant selection bias. The CRY Centre for Cardiac Pathology at the Royal Brompton Hospital is an internationally recognised cardiac pathology centre in the UK, where the hearts of many young athletes are commonly referred, especially when the findings are ambiguous and no clear cause of death can be established by the local pathologist. It is therefore highly probable that cardiac anomalies such as coronary artery atherosclerosis, which can be easily identified, and cardiomyopathies such as HCM, which have been well characterised, are under-represented in this cohort. Similarly the prevalence of less well-defined entities such as idiopathic left ventricular hypertrophy and a morphologically normal heart are likely to represent an overestimate. A national database on SCD in young athletes needs to be established in order to obtain more accurate information in the future.
Acknowledgments
We acknowledge the charitable organisation, Cardiac Risk in the Young (CRY) for funding, SN, MP and providing financial support for the study. We also acknowledge the support laboratory staff of the histopathology department at the Royal Brompton Hospital and Dr Thomas Grüning for proof reading and academic assistance with the manuscript.
REFERENCES
Footnotes
Funding The study was part funded by the charitable organisation Cardiac Risk in the Young. SN is funded by a research fellow grant by CRY. MP is funded by a junior research fellowship grant by CRY.
Competing interests SS is consultant cardiologist and trustee to the charitable organisation Cardiac Risk in the Young (CRY) and has received research grants from CRY. GW is CRY trustee and has received research grants from CRY. MS has received a research grant from CRY. SN and MP are funded by research fellowship grants from CRY.
Ethics approval Brompton, Harefield and National Heart and Lung Institute: Ref 07/Q040.
Authorship and contributorship: SN, conception and design, literature search, analysis and interpretation of data and drafting the article; SS, SD, MP, GW, MNS, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content; MNS, additionally, performed post mortems. All the authors approved the final version to be published.