Article Text
Abstract
Aim To establish a reference range for oxygen saturation (SpO2) in well preterm infants to guide home oxygen therapy using a pulse oximeter and Pulse Oximetry Data Analysis Software (PODS).
Methods SpO2 and heart-rate profiles of healthy preterm infants receiving mechanical ventilation for less than 6 h and supplemental oxygen for less than 48 h were monitored using a pulse oximeter. The stored data were downloaded from the monitor to a personal computer as individual files. Each infant's files of SpO2 were subsequently displayed in graphic form, and a reference range was constructed using dedicated software, PODS.
Results 43 infants were studied. The median value of all infants mean SpO2 values was 95% (range 92–99%). The median duration of saturations less than 85% and between 85% and 90 % were 1% and 2% respectively. Using the study group median, 5th and 95th percentiles, a cumulative frequency curve of time against SpO2 value was constructed (representing the reference range of SpO2 profiles in healthy preterm infants).
Conclusion The SpO2 reference range can be used as an easy and practical guide to compare SpO2 profiles of infants on home oxygen therapy and guide their oxygen therapy.
Statistics from Altmetric.com
Background
Significant improvements in obstetric and neonatal care have resulted in improved survival rates of very-low-birthweight infants. Despite these improvements, chronic lung disease (CLD) remains a significant problem, especially among the very-low-birthweight infants resulting in prolonged hospital stay, discharged home with oxygen therapy and frequent respiratory problems in the first year of life.1,–,3
Adequate oxygen treatment in babies with CLD may prevent pulmonary hypertension but also promotes growth,4 but oxygen toxicity may also increase retinopathy of prematurity (ROP), bronchopulmonary dysplasia and periventicular leucomalacia.5 However, uncertainty continues as to the most appropriate ranges to maintain oxygen levels for term or preterm infants or a threshold value below which oxygen should be administered.6 7 A recently published randomised trial concluded that a lower target range of oxygen saturations (85–91%) compared with a higher range (91–95%) did not significantly decrease the composite outcome of severe ROP or death but resulted in an increase in mortality and substantial decrease in severe ROP among survivors.5 BOOST II-UK is an ongoing randomised trial investigating the optimal blood oxygen saturation in preterm babies.
What is already known on this topic
▶ Oxygen saturations (SpO2) are used to guide home oxygen therapy.
▶ Home oxygen is often required in infants with chronic lung disease.
What this study adds
▶ Provides a reference range for SpO2 in well preterm infants to guide home oxygen therapy using Nellcor pulse oximeter.
▶ Pulse Oximetry Data Analysis Software adds a visual reference display for continuous oxygen saturation monitoring.
Some stable premature infants with CLD who continue to require oxygen after term can be managed on home oxygen. This can result in early discharge and may facilitate parent–infant bonding, optimise the infant's development and be more cost-effective.
However, it is important to ensure that babies with CLD needing supplemental oxygen are monitored appropriately to help to prevent chronic intermittent hypoxia and avoid excess oxygen, which may be harmful. There are no agreed standards for the process of withdrawing oxygen from babies. Prolonged pulse oximetry is commonly used to determine the appropriateness of withdrawal of supplemental oxygen.
Using plethsmography and graphic analysis techniques, some studies have attempted to define a normal reference range of SpO2.8 9 Although this is undoubtedly a thorough methodology, it is less practical for routine use in neonatal units. Furthermore, a limitation of using internal oximeter data storage modes is that it involves intermittent sampling or averaging techniques which may lead erroneously to lower saturations owing to movement artefact.
Using a data-logging device and dedicated software, we have already produced a reference range for oxygen saturations in a small group of preterm infants using SpO2 profiles.10 This reference range applies to Ohmeda pulse oximeters (Ohmeda, Boulder, Colorado) and can be used in a clinical setting. However, another group of pulse oximeters (made by Nellcor; Tyco Healthcare Group LP, Pleasanton, California) are also in routine clinical use. They have a different algorithm for calculating the oxygen dissociation curve and tend to produce a reading that is higher than the Ohmeda oximeters by approximately 2%. A reference range for use in children with CLD similar to that which we have previously produced does not exist for the Nellcor oximeter.
The aim of this study was to construct a simple reference range of SpO2 in healthy preterm infants using Nellcor monitors. Such reference curves are helpful in guiding the management of oxygen treatment in preterm infants with CLD.
Methods
This was a descriptive prospective study. Infants were recruited to the study at Liverpool Women's Hospital, UK, after local regional ethics committee approval had been granted. Preterm infants who received mechanical ventilation for less than 6 h and supplemental oxygen for less than 48 h were recruited. Exclusion criteria included infants with congenital anomalies and cardiorespiratory disorders. Parental consent was obtained. Gestational age was assessed from maternal dates and/or early ultrasound examination. Demographic data were collected from maternal and infant case records. All infants had their oxygen saturations measured for a continuous period of 4 h following a feed, while asleep or during a period of quite wakefulness. The infant was left undisturbed during this period.
Nellcor N 595 pulse oximeter was used for recording arterial oxygen saturations and heart rate. The saturation probe, Oximax-max-N sensor was placed postductally on the infant's foot or hand, whichever provided an optimal signal. The stored data were downloaded from the Nellcor monitor to a personal computer and saved as individual files. A dedicated software, Pulse Oximetry Data Analysis Software (PODS) developed at the Department of Medical Physics and Clinical Engineering, Royal Liverpool University Hospital, was used to analyse the data.
PODS (http://www.pods-software.com) is a bespoke software package written in Microsoft VB version 6.0. The software downloads pulse oximetry data stored in non-volatile memory via a serial link, displays trend graphs, histograms and cumulative distribution graphs, and produces a numerical statistical analysis summary. One of the main features of PODS that other similar software packages do not have is the cumulative distribution analysis. The cumulative distribution function (F) is the probability that the saturation value (S) takes a value less than or equal to x:

where 85≤x≤100.
SpO2 values below 85% are not binned any further. For example, if a child spends 30 min in a 6 h recording at various levels that are ≤85%, then the leftmost point of the curve corresponding to 85% will have a y value of 8.3% (0.5/6×100%).
This statistic enables the clinician to determine what proportion of time the patient was hypoxic during the study period. The software allows the user to define a number of control groups according to age and pathology, and update the normative range for each group dynamically. It displays expected and lowest cumulative distribution curves (representing the fifth and 50th percentiles respectively) of a matching control group superimposed on the cumulative distribution curve of the patient record. The clinical studies presented in the results section have been referenced to the normal range produced in this study. Finally, PODS software has been CE-marked under the Medical Device Directive MDD 93/42/EEC as a Medical Device Class IIa (http://www.mhra.gov.uk/Howweregulate/Devices/MedicalDevicesDirective/).
In order to obtain this mark, the software went through formal risk analysis, validation and verification procedures, and the technical file was inspected by a notified body.
Each infant's SpO2 and heart rate profiles were then displayed in graphic form and were analysed using the dedicated software. After exclusion of obvious movement artefact (defined as deflection to zero of both SpO2 and heart rate tracings), the following analysis was performed using the raw data in a standardised manner by the same observer: (1) the total duration of the recording; (2) the median saturation; (3) the median heart rate; (4) the time spent below 85% and between 85% and 90% saturation; and (5) the cumulative time (expressed as a percentage of total time) spent below a range of individual SpO2 levels between 85% and 100%. A cumulative frequency curve of time against SpO2 was then constructed using the study group median and 5th and 95th percentiles. This curve represented the normal reference range of SpO2 profiles for healthy preterm infants. Descriptive statistics and graphs were generated using Microsoft Excel software.
Results
Forty-three preterm infants were studied, 17 male and 26 female. None of the infants were receiving methylxanthines, and all were breathing spontaneously in air at the time of the study. The median gestational age of the study group was 33 weeks (range 29–36). The median age at the time of study was 14 days (IQR 6–25).
The infants were monitored for a median duration of 4.1 h (range 3.5–6.6 h). The median heart rate was 150 beats per minute (range 116–168). The median value of all infants' median SpO2 values was 95% (range 92–99%). The median duration of saturations less than 85% and between 85% and 90% was 1% (range 1–4%) and 2% (range 1–5%) respectively. Table 1 shows the cumulative figures for healthy preterm infants as a percentage of the time spent at or below each oxygen saturation value.
Cumulative figures for healthy preterm infants as a percentage of time spent at or below each oxygen saturation value
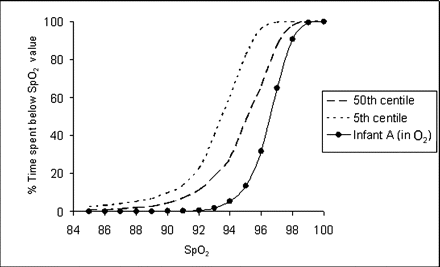
Figure 1 shows the graph with the 5th and 50th centile for the cumulative frequency for SpO2 for healthy preterm infants. Using this graph, we can compare the individual babies' SPO2 profiles against these normal values.
Oxygen saturation (Spo2) profile in healthy preterm infants.
Figures 2, 3 are examples of individual SpO2 profiles (along with existing reference ranges) of a 26-week-gestation baby who initially was receiving 0.75 l/min of oxygen and then was nursed in air respectively. In figure 2, the study infant's curve is to the right of the normal curves, suggesting that the oxygen can be weaned further.
Oxygen saturation (Spo2) profile for a 26-week infant (infant A) when oxygen was reduced from 1 l/min to 0.75 l/min.
Oxygen saturation (Spo2) profile for a 26-week infant (infant A): study in air (oxygen discontinued).
Discussion
By using the data-logging device and dedicated software, PODS, we have constructed normal SpO2 profiles in healthy preterms. The median value of all infants' median SpO2 values was 95% (range 92–99%). The median duration of saturations less than 85% and between 85% and 90% was 1% (range 1–4%) and 2% (range 1–5%) respectively. This applies to the Nellcor N595 pulse oximeters. We have already produced a reference range for SpO2 in a small group of preterm infants that apply to Ohmeda pulse oximeters and can be used in a clinical setting.10 It is known that different makes of pulse oximeters use different algorithms for calculating the oxygen dissociation curve. The Nellcor pulse oximeters tend to produce a reading that is higher than the Ohmeda pulse oximeters by approximately 2%.11 To our knowledge, this is the first study to construct a normal SpO2 profile in healthy preterm infants that apply to Nellcor N595 pulse oximeters that are currently in clinical use.
The median of mean saturations reported in this study was 95%, which is lower than what we reported previously, 97% using the Ohmeda monitors.10 Another study reported a median baseline SpO2 value of 99.5% (range 88.7–100%) using the Nellcor N200 pulse oximeter.8 This difference noted between the studies could be due to the different software versions and may also be related methods relating to artefact exclusion or could be due to chance. Therefore, it is important to re-evaluate any instrument with new software.
We have shown that the median time spent below 85% and between 85% and 90% saturations was 1% and 2% respectively. Using the group median, fifth and 95th percentiles, a cumulative frequency curve of time against SpO2 was constructed. The cumulative frequency curve of oxygen saturations represents the range of SpO2 profiles in healthy preterm infants. This curve may be helpful in guiding supplemental oxygen therapy in babies with CLD such as the examples in figures 2, 3 as they are practical, easy to interpret, user-friendly and more informative than using a single figure of mean (SD) SpO2. By superimposing an individual SpO2 profile on the group cumulative frequency curve, any deviation from the normal SpO2 profile pattern can be assessed. The shift of the infants profile to the left indicates that a greater amount of time is spent at levels below normal values, and this might indicate a requirement for additional oxygen. These SpO2 curves could also be used in monitoring babies on home oxygen.
While we acknowledge that it may be desirable in some situations to record simultaneous heart rate, saturation and respiratory tracings, we feel that this is not practical in home care settings and not relevant to detecting mild persistent hypoxia. We excluded all major artefacts but cannot be certain about minor artefacts (which may have marginally lowered the overall mean SPO2).
In this study, infants were monitored for a median period of 4.1 h during a quiet phase. By doing this, we have eliminated any movement artefacts related to feeding and handling.12 A more prolonged period of recording would involve feeding and handling, and a period of wakefulness that may affect SpO2 levels.9 11 13 Thus, our study with a recording of 4 h permitted a period of study that was reasonable and also practical.
In summary, we have constructed and described a reference range for SPO2 profiles in healthy preterm babies that can be applied to Nellcor pulse oximeters. It is practical and easy to use. Comparing this with SpO2 profiles of individual babies may help in guiding the management of babies with CLD who are receiving supplemental oxygen.
References
Footnotes
-
Competing interests The software is available commercially following consultation with the NHS regional innovation hub TrusTECH. It was agreed that the IP is shared between the Royal Liverpool and Broadgreen University Hospitals NHS Trust, Liverpool Women's NHS Foundation Trust and Royal Liverpool Children's NHS Trust.
-
Ethics approval Ethics approval was provided by Liverpool (Paediatric) LREC.
-
Provenance and peer review Not commissioned; externally peer reviewed.