Abstract
Purpose
The present study was performed to culture human fetal ova to determine whether they can be matured and cryopreserved using ultrarapid freezing
Methods
Thirty-three pairs of fetal ovaries were obtained from fetuses of 16–20 weeks' gestation following elective abortion. Ovarian tissues were minced into approximately 1-mm sizes and cultured in Waymouth media either before or after ultrarapid freezing. The Waymouth medium was supplemented with 15% (v/v) fetal bovine serum, 0.03 IU/ml FSH and 35 ng/ml insulin. The tissue was cultured at 37‡C in 5% CO2 in air for 5– 25 days in Falcon dishes and 30–40 days in Costar Transwell-COL membranes prior to induction of final maturation in the presence of LH and human follicular fluid. Minced tissues were also frozen by ultrarapid freezing in M199 with 4.2M dimethylsulfoxide (DMSO) and 0.35M sucrose and plunged directly into liquid nitrogen. For thawing, the straws were plunged into a 37‡C water bath for 5 s. The contents were then expelled and diluted 1∶5 with thawing medium containing 0.42 M sucrose. After washing the thawed tissues were cultured as described for the fresh tissues.
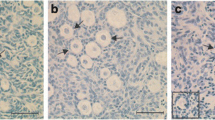
Results
Patches of monolayer consisting of fibroblasts had formed within 2– 3 days of culture of fresh tissues. After 1 week of culture, follicles separated out from the ovarian tissue but remained attached to the monolayer. The maximal number of follicles separating out from the tissue appeared about 1 week after initiating the culture (154 follicles per 10 fields at Day 5 and 61 and Day 25). After 40 days of culture in Costar dishes, 34% of the ova reached a diameter of more than 80Μm, which was significantly higher than at the beginning of culture (6%;P < 0.05). Among these ova, 34% were found to be surrounded by the zona pellucida, which was not observed at the beginning of culture. Following induction of final maturation, extrusion of the first polar body was noted in 25% of ova grown in Costar dishes for 40 days. Twelve percent of the oocytes showed the first polar body when they were grown in Costar dishes for less than 30 days. For frozen-thawed tissues, 14% of minced ovarian tissues displayed central necrosis immediately after thawing. Following digestion and Trypan blue staining, 75% of ova and 85% of somatic cells survived ultrarapid freezing. Nineteen percent of the ova which have been cultured as described for fresh tissues displayed extrusion of the first polar body, comparing favorably with the 25% maturation rate observed with the fresh tissue (P>0.05).
Conclusion
This study demonstrates that morphologically normal, mature human ova can be obtained from primordial follicles in vitro development. Using a simple, quick ultrarapid freezing method, human fetal ova can be cryopreserved in the form of minced tissue without significantly compromising their ability to grow in vitro.
Similar content being viewed by others
References
Ohno S, Mikino S, Kaplan W, Konosita R: Female germ cells in man. Exp Cell Res 1961;24:106–109
Baker TG: Quantitation and cytological study of germ cells in human ovaries. Proc R Soc Ser B 1963;158:417–433
Baker TG, Franchi LL: The fine structure of oogonia and oocytes in human ovaries. J Cell Sci 1967;2:213–232
Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK: Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril 1991;55:109–113
Shaw JM, Oranratnachai, Trounson A: Cryopreservation of oocytes and embryos.In Handbook of in vitro fertilization, A Trounson, D Gardner (eds). 1993, pp 213–236
Shaw JM, Diotallevi L, Trounson AO: A simple rapid dimethyl sulphoxide freezing technique for the cryopreservation of one-cell to blastocyst stage preimplantation mouse embryo. Reprod Fert Dev 1991;3:3–12
Eshkol A, Lunenfeld B, Peters H: Ovarian development in infant mice: Dependence on gonadotrophic hormones.In Gonadotrophins and Ovarian Development, Burt WR, Crooke AC, Ryle M (eds.), Edinburgh, Churchill and Livingstone, 1970, pp 249–258
Richardson SJ, Senikas V, Nelson JF: Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 1987; 65:1231–1237
Gougeon A: Dynamics of human follicular growth: A morphologic perspective. In EY Adashi, PCK Leung (eds.), The Ovary. New York, Raven Press, 1993, pp 21–39
Eppig JJ, Schroeder AC: Capacity of mouse oocytes from pre-antral follicles to undergo embryogenesis and development to live young after growth, maturation and fertilization in vitro. Biol Reprod 1989;42:268–276
Eppig JJ, Wigglesworth K, O'Brien MJ: Comparison of embryonic developmental competence of mouse oocytes grown with and without serum. Mol Reprod Dev 1992;32:33–40
Qvist R, Blackwell LF, Bourne H, Brown JB: Development of mouse ovarian follicles from primary to preovulatory stages in vitro. J Reprod Fertil 1990;89:169–180
Glenister PH, Wood MJ, Kirby C, Whittingham DG: Incidence of chromosome anomalies in first cleavage mouse embryos obtained from frozen-thawed oocytes fertilized in vitro. Gamete Res 1987;16:205–216
Johnson MH, Pickering SJ, George MA: The influence of cooling on the properties of the zona pellucida of the mouse oocyte. Hum Reprod 1988;3:383–387
Trounson A, Kirby C: Problems in the cryopreservation of unfertilized eggs by slow cooling in dimentyl sulfoxide. Fertil Steril 1989;52:778–786
Vincent C, Pickering SJ, Johnson MH, Quick SJ: Dimethylsulfoxide affects the organization of microfilaments in the mouse oocyte. Mol Reprod Dev 1990;26:227–235
George MA, Johnson MH, Howlett SK: Assessment of the developmental potential of frozen-thawed mouse oocytes. Hum Reprod 1994;9:130–136
Carroll J, Gosden RG, Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod 1993;8:1163–1167
Parrott DMV: The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil 1960;1:230–241
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, J., Liu, J., Xu, K.P. et al. Extracorporeal development and ultrarapid freezing of human fetal ova. J Assist Reprod Genet 12, 361–368 (1995). https://doi.org/10.1007/BF02215727
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02215727